Abstract
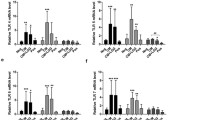
Lactoferrin possesses a wide range of immunomodulatory activities, including promotion of the delayed type hypersensitivity response (DTH) towards BCG (Bacillus Calmette Guerin) antigens. Addition of Lactoferrin as an adjuvant to the BCG vaccine was previously demonstrated to augment protection against subsequent mycobacterial challenge, with concomitant development of a strong T cell helper type 1 (TH1) immunity. Because generation of TH1 immunity is in large part dependent on the balance of monocytic pro- and anti-inflammatory cytokines, the effect of Lactoferrin on leukocytes was investigated. Lactoferrin enhanced proinflammatory responses in a dose-dependant manner from splenocyte and adherent (F4/80+) splenocyte populations, bone marrow derived monocytes (BMM), and J774A.1 cultured cells. In all scenarios tested, Lactoferrin induced a strong increase in the ratio of IL-12:IL-10 production from LPS stimulated cells. Examination of Lactoferrin effects on BCG infected J774A.1 cells and on BMM revealed similar immunomodulatory effects, with particularly strong increase in IL-12 production. Furthermore, immunization of mice with BCG admixed with Lactoferrin led to increased generation of CD4+ cells expressing IFN-γ upon restimulation with BCG antigens. These results provide molecular evidence to support the role of Lactoferrin as an adjuvant candidate to augment development of DTH response to vaccine antigens.




Similar content being viewed by others
References
Actor JK, Hwang SA, Olsen M, Zimecki M, Hunter RL Jr, Kruzel ML (2002) Lactoferrin immunomodulation of DTH response in mice. Int Immunopharmacol 2:475–486
Actor JK, Olsen M, Jagannath C, Hunter RL (1999) Relationship of survival, organism containment, and granuloma formation in acute murine tuberculosis. J Interferon Cytokine Res 19:1183–1193
Baveye S, Elass E, Fernig DG, Blanquart C, Mazurier J, Legrand D (2000) Human lactoferrin interacts with soluble CD14 and inhibits expression of endothelial adhesion molecules, E-selectin and ICAM-1, induced by the CD14-lipopolysaccharide complex. Infect Immun 68:6519–6525
Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, Britton WJ (1999) Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol 162:3504–3511
Behr MA (2002) BCG-different strains, different vaccines? Lancet Infect Dis 2:86–92
Brewer TF (2000) Preventing tuberculosis with bacillus Calmette-Guerin vaccine: a meta-analysis of the literature. Clin Infect Dis 31(Suppl 3):S64–67
Chen C, Boylan MT, Evans CA, Whetton AD, Wright EG (2005) Application of two-dimensional difference gel electrophoresis to studying bone marrow macrophages and their in vivo responses to ionizing radiation. J Proteome Res 4:1371–1380
Chodaczek G, Zimecki M, Lukasiewicz J, Lugowski C (2006) A complex of lactoferrin with monophosphoryl lipid A is an efficient adjuvant of the humoral and cellular immune response in mice. Med Microbiol Immunol (Berl) 195:207–216
Choe YH, Lee SW (1999) Effect of lactoferrin on the production of tumor necrosis factor-alpha and nitric oxide. J Cell Biochem 76:30–36
Cooper AM, Magram J, Ferrante J, Orme IM (1997) Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med 186:39–45
Damiens E, Mazurier J, Yazidi I el, Masson M, Duthille I, Spik G, Boilly-Marer Y (1998) Effects of human lactoferrin on NK cell cytotoxicity against haematopoietic and epithelial tumour cells. Biochim Biophys Acta 1402:277–287
Dhennin-Duthille I, Masson M, Damiens E, Fillebeen C, Spik G, Mazurier J (2000) Lactoferrin upregulates the expression of CD4 antigen through the stimulation of the mitogen-activated protein kinase in the human lymphoblastic T Jurkat cell line. J Cell Biochem 79:583–593
Diehl S, Rincon M (2002) The two faces of IL-6 on Th1/Th2 differentiation. Mol Immunol 39:531–536
Edde L, Hipolito RB, Hwang FF, Headon DR, Shalwitz RA, Sherman MP (2001) Lactoferrin protects neonatal rats from gut-related systemic infection. Am J Physiol Gastrointest Liver Physiol 281:G1140–1150
Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias PS, Mazurier J, Spik G (1998) Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun 66:486–491
Fine PE (1995) Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339–1345
Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A (1991) IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 146:3444–3451
Fischer R, Debbabi H, Dubarry M, Boyaka P, Tome D (2006) Regulation of physiological and pathological Th1 and Th2 responses by lactoferrin. Biochem Cell Biol 84:303–311
Flynn JL, Chan J (2001) Immunology of tuberculosis. Annu Rev Immunol 19:93–129
Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, Schreiber R, Mak TW, Bloom BR (1995) Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity 2:561–572
Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom BR (1995) IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol 155:2515–2524
Gately MK, Renzetti LM, Magram J, Stern AS, Adorini L, Gubler U, Presky DH (1998) The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol 16:495–521
Hodge-Dufour J, Marino MW, Horton MR, Jungbluth A, Burdick MD, Strieter RM, Noble PW, Hunter CA, Pure E (1998) Inhibition of interferon gamma induced interleukin 12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factor. Proc Natl Acad Sci USA 95:13806–13811
Hovav AH, Mullerad J, Davidovitch L, Fishman Y, Bigi F, Cataldi A, Bercovier H (2003) The Mycobacterium tuberculosis recombinant 27-kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protection. Infect Immun 71:3146–3154
Hwang SA, Kruzel ML, Actor JK (2005) Lactoferrin augments BCG vaccine efficacy to generate T helper response and subsequent protection against challenge with virulent Mycobacterium tuberculosis. Int Immunopharmacol 5:591–599
Indrigo J, Hunter RL Jr, Actor JK (2003) Cord factor trehalose 6,6'-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology 149:2049–2059
Kocieba M, Zimecki M, Kruzel M, Actor J (2002) The adjuvant activity of lactoferrin in the generation of DTH to ovalbumin can be inhibited by bovine serum albumin bearing alpha-d-mannopyranosyl residues. Cell Mol Biol Lett 7:1131–1136
Kruzel ML, Bacsi A, Choudhury B, Sur S, Boldogh I (2006) Lactoferrin decreases pollen antigen-induced allergic airway inflammation in a murine model of asthma. Immunology
Kruzel ML, Harari Y, Mailman D, Actor JK, Zimecki M (2002) Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin Exp Immunol 130:25–31
Kruzel ML, Zimecki M (2002) Lactoferrin and immunologic dissonance: clinical implications. Arch Immunol Ther Exp (Warsz) 50:399–410
Ma X, Sun J, Papasavvas E, Riemann H, Robertson S, Marshall J, Bailer RT, Moore A, Donnelly RP, Trinchieri G, Montaner LJ (2000) Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J Immunol 164:1722–1729
Miyauchi H, Hashimoto S, Nakajima M, Shinoda I, Fukuwatari Y, Hayasawa H (1998) Bovine lactoferrin stimulates the phagocytic activity of human neutrophils: identification of its active domain. Cell Immunol 187:34–37
Moreira AL, Tsenova L, Aman MH, Bekker LG, Freeman S, Mangaliso B, Schroder U, Jagirdar J, Rom WN, Tovey MG, Freedman VH, Kaplan G (2002) Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect Immun 70:2100–2107
Mosmann TR, Moore KW (1991) The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today 12:A49–53
Murphy EE, Terres G, Macatonia SE, Hsieh CS, Mattson J, Lanier L, Wysocka M, Trinchieri G, Murphy K, O’Garra A (1994) B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med 180:223–231
Na YJ, Han SB, Kang JS, Yoon YD, Park SK, Kim HM, Yang KH, Joe CO (2004) Lactoferrin works as a new LPS-binding protein in inflammatory activation of macrophages. Int Immunopharmacol 4:1187–1199
Roseanu A, Chelu F, Trif M, Motas C, Brock JH (2000) Inhibition of binding of lactoferrin to the human promonocyte cell line THP-1 by heparin: the role of cell surface sulphated molecules. Biochim Biophys Acta 1475:35–38
Saunders BM, Frank AA, Orme IM, Cooper AM (2000) Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect Immun 68:3322–3326
Schmitt E, Hoehn P, Huels C, Goedert S, Palm N, Rude E, Germann T (1994) T helper type 1 development of naive CD4+ T cells requires the coordinate action of interleukin-12 and interferon-gamma and is inhibited by transforming growth factor-beta. Eur J Immunol 24:793–798
Seder RA, Gazzinelli R, Sher A, Paul WE (1993) Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA 90:10188–10192
Shau H, Kim A, Golub SH (1992) Modulation of natural killer and lymphokine-activated killer cell cytotoxicity by lactoferrin. J Leukoc Biol 51:343–349
Sorimachi K, Akimoto K, Hattori Y, Ieiri T, Niwa A (1997) Activation of macrophages by lactoferrin: secretion of TNF-alpha, IL-8 and NO. Biochem Mol Biol Int 43:79–87
Suzuki YA, Lonnerdal B (2002) Characterization of mammalian receptors for lactoferrin. Biochem Cell Biol 80:75–80
Teraguchi S, Wakabayashi H, Kuwata H, Yamauchi K, Tamura Y (2004) Protection against infections by oral lactoferrin: evaluation in animal models. Biometals 17:231–234
Umemura M, Nishimura H, Saito K, Yajima T, Matsuzaki G, Mizuno S, Sugawara I, Yoshikai Y (2003) Interleukin-15 as an immune adjuvant to increase the efficacy of Mycobacterium bovis bacillus Calmette-Guerin vaccination. Infect Immun 71:6045–6048
Wakabayashi H, Kurokawa M, Shin K, Teraguchi S, Tamura Y, Shiraki K (2004) Oral lactoferrin prevents body weight loss and increases cytokine responses during herpes simplex virus type 1 infection of mice. Biosci Biotechnol Biochem 68:537–544
Wakabayashi H, Takakura N, Yamauchi K, Tamura Y (2006) Modulation of immunity-related gene expression in small intestines of mice by oral administration of lactoferrin. Clin Vaccine Immunol 13:239–245
Zganiacz A, Santosuosso M, Wang J, Yang T, Chen L, Anzulovic M, Alexander S, Gicquel B, Wan Y, Bramson J, Inman M, Xing Z (2004) TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J Clin Invest 113:401–413
Zimecki M, Kocieba M, Kruzel M (2002) Immunoregulatory activities of lactoferrin in the delayed type hypersensitivity in mice are mediated by a receptor with affinity to mannose. Immunobiology 205:120–131
Zimecki M, Kruzel ML (2000) Systemic or local co-administration of lactoferrin with sensitizing dose of antigen enhances delayed type hypersensitivity in mice. Immunol Lett 74:183–188
Zimecki M, Mazurier J, Spik G, Kapp JA (1995) Human lactoferrin induces phenotypic and functional changes in murine splenic B cells. Immunology 86:122–127
Acknowledgments
This work was accomplished with support from NIH grant R41AI51050-01 and R42-AI051050-02. We thank Michal Zimecki Ph.D. (Department of Experimental Therapy, Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland) and Robert L. Hunter, M.D., Ph.D. (University of Texas-Houston Medical School, Department of Pathology) for helpful conversations and data discussion, and Margaret Olsen for assistance and technical expertise.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hwang, SA., Wilk, K.M., Bangale, Y.A. et al. Lactoferrin modulation of IL-12 and IL-10 response from activated murine leukocytes. Med Microbiol Immunol 196, 171–180 (2007). https://doi.org/10.1007/s00430-007-0041-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-007-0041-6