Abstract
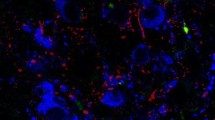
Morphological and pharmacological studies indicate that hypothalamic neuropeptide Y (NPY) and proopiomelanocortin (POMC) neurons communicate with each other in rats and regulate a variety of hypothalamic and extrahypothalamic functions. Indeed, electron microscopic studies revealed NPY-immunoreactive (NPI-IR) synapses on β-endorphin-IR neurons in the hypothalamus. However, no such connections have been reported in humans. Here, we studied the putative NPY–β-endorphin associations with high-resolution light microscopic double-label immunocytochemistry in the human hypothalamus. The majority of β-endorphin-IR perikarya appear to be innervated by abutting NPY-IR fibers in the infundibulum/median eminence, receiving more than 6 contacts (38% of the counted neurons) or three to six contacts (42% of the counted neurons). The rest of the β-endorphin-IR neurons are lightly innervated by NPY fibers (14%, one–three contacts) or do not receive any detectable NPY-IR axon varicosities (6% of the counted neurons). Since β-endorphin is cleaved from the proopiomelanocortin (POMC) precursor, the NPY–β-endorphin connections also provide the foundation for NPY–α-MSH and NPY–ACTH connections and their subsequent physiology. The close anatomical connections between NPY-IR nerve terminals and β-endorphin-IR neurons reported herein may represent functional synapses and provide the foundation for NPY-stimulated β-endorphin release. By interacting with β-endorphin, NPY may have a more widespread regulatory capacity than acting alone on different neurotransmitter systems.


Similar content being viewed by others
Data availability
The data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adam TC, Epel ES (2007) Stress, eating and the reward system. Physiol Behav 91:449–458
Braak H, Braak E (1987) The hypothalamus of the human adult: chiasmatic region. Anat Embryol (berl) 175:315–330
Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T (1998) The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A 95:15043–15048
Bugnon C, Bloch B, Lenys D, Gouget A, Fellmann D (1979) Comparative study of the neuronal populations containing beta-endorphin, corticotropin and dopamine in the arcuate nucleus of the rat hypothalamus. Neurosci Lett 14:43–48
Chronwall BM (1985) Anatomy and physiology of the neuroendocrine arcuate nucleus. Peptides 6(Suppl 2):1–11
Crown A, Clifton DK, Steiner RA (2007) Neuropeptide signaling in the integration of metabolism and reproduction. Neuroendocrinology 86:175–182
Csiffary A, Gorcs TJ, Palkovits M (1990) Neuropeptide Y innervation of ACTH-immunoreactive neurons in the arcuate nucleus of rats: a correlated light and electron microscopic double immunolabeling study. Brain Res 506:215–222
Deltondo J, Por I, Hu W et al (2008) Associations between the human growth hormone-releasing hormone- and neuropeptide-Y-immunoreactive systems in the human diencephalon: a possible morphological substrate of the impact of stress on growth. Neuroscience 153:1146–1152
Dierschke DJ (1985) Temperature changes suggestive of hot flushes in rhesus monkeys: preliminary observations. J Med Primatol 14:271–280
Dudas B, Merchenthaler I (2004) Close anatomical associations between beta-endorphin and luteinizing hormone-releasing hormone neuronal systems in the human diencephalon. Neuroscience 124:221–229
Dudas B, Merchenthaler I (2006) Three-dimensional representation of the neurotransmitter systems of the human hypothalamus: inputs of the gonadotrophin hormone-releasing hormone neuronal system. J Neuroendocrinol 18:79–95
Dudas B, Mihaly A, Merchenthaler I (2000) topography and associations of luteinizing hormone-releasing hormone and neuropeptide Y-immunoreactive neuronal systems in the human diencephalon. J Comp Neurol 427:593–603
Escobar CM, Krajewski SJ, Sandoval-Guzman T, Voytko ML, Rance NE (2004) Neuropeptide Y gene expression is increased in the hypothalamus of older women. J Clin Endocrinol Metab 89:2338–2343
Fu LY, Van Den Pol AN (2010) Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 30:10205–10219
Fuxe K, Tinner B, Caberlotto L, Bunnemann B, Agnati LF (1997) NPY Y1 receptor like immunoreactivity exists in a subpopulation of beta-endorphin immunoreactive nerve cells in the arcuate nucleus: a double immunolabelling analysis in the rat. Neurosci Lett 225:49–52
Gallyas F, Merchenthaler I (1988) Copper-H2O2 oxidation strikingly improves silver intensification of the nickel-diaminobenzidine (Ni-Dab) end-product of the peroxidase reaction. J Histochem Cytochem 36:807–810
Gallyas F, Gorcs T, Merchenthaler I (1982) High-grade intensification of the end-product of the diaminobenzidine reaction for peroxidase histochemistry. J Histochem Cytochem 30:183–184
Hahn TM, Breininger JF, Baskin DG, Schwartz MW (1998) Coexpression of AGRP and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1:271–272
Hill JW (2010) Gene expression and the control of food intake by hypothalamic POMC/Cart neurons. Open Neuroendocrinol J 3:21–27
Hill JW, Elias CF, Fukuda M et al (2010) Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 11:286–297
Horvath TL, Naftolin F, Kalra SP, Leranth C (1992) Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology 131:2461–2467
Hrabovszky E, Sipos MT, Molnar CS et al (2012) Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology 153:4978–4989
Kageyama H, Takenoya F, Hirako S et al (2012) Neuronal circuits involving neuropeptide Y in hypothalamic arcuate nucleus-mediated feeding regulation. Neuropeptides 46:285–289
Kalra SP, Crowley WR (1992) Neuropeptide Y: a novel neuroendocrine peptide in the control of pituitary hormone secretion, and its relation to luteinizing hormone. Front Neuroendocrinol 13:1–46
Kalra SP, Allen LG, Sahu A, Kalra PS, Crowley WR (1988) Gonadal steroids and neuropeptide Y-opioid-LHRH axis: interactions and diversities. J Steroid Biochem 30:185–193
Kalra SP, Sahu A, Kalra PS, Crowley WR (1990) Hypothalamic neuropeptide Y: a circuit in the regulation of gonadotropin secretion and feeding behavior. Ann N Y Acad Sci 611:273–283
Khachaturian H, Lewis ME, Tsou K, Watson SJ (1985) Beta-endorphin, alpha-MSH and related peptides. In: Björklund A, Hökfelt T (eds) Gaba and neuropeptides in the CNS. Elsevier, Amsterdam, pp 216–272
Klenke U, Constantin S, Wray S (2010) Neuropeptide Y directly inhibits neuronal activity in a subpopulation of gonadotropin-releasing hormone-1 neurons via Y1 receptors. Endocrinology 151:2736–2746
Ko L, Rotoli G, Grignol G, Hu W, Merchenthaler I, Dudas B (2011) A putative morphological substrate of the catecholamine-influenced neuropeptide Y (NPY) release in the human hypothalamus. Neuropeptides 45:197–203
Li C, Chen P, Smith MS (1999) Morphological evidence for direct interaction between arcuate nucleus neuropeptide Y (NPY) neurons and gonadotropin-releasing hormone neurons and the possible involvement of NPY Y1 receptors. Endocrinology 140:5382–5390
Louis GW, Greenwald-Yarnell M, Phillips R, Coolen LM, Lehman MN, Myers MG Jr (2011) Molecular mapping of the neural pathways linking leptin to the neuroendocrine reproductive axis. Endocrinology 152:2302–2310
Maniam J, Morris MJ (2012) The link between stress and feeding behaviour. Neuropharmacology 63:97–110
Mcdonald J, Calka J (1994) Relationship between neuropeptide Y and luteinizing-hormone-releasing hormone immunoreactivities in the hypothalamus and preoptic region. Acta Anat (basel) 151:171–179
Menyhert J, Wittmann G, Lechan RM, Keller E, Liposits Z, Fekete C (2007) Cocaine- and amphetamine-regulated transcript (Cart) is colocalized with the orexigenic neuropeptide Y and agouti-related protein and absent from the anorexigenic alpha-melanocyte-stimulating hormone neurons in the infundibular nucleus of the human hypothalamus. Endocrinology 148:4276–4281
Merchenthaler I, Rotoli G, Grignol G, Dudas B (2010) Intimate associations between the neuropeptide Y system and the galanin-immunoreactive neurons in the human diencephalon. Neuroscience 170:839–845
Morton GJ, Schwartz MW (2001) The NPY/AGRP neuron and energy homeostasis. Int J Obes Relat Metab Disord 25(Suppl 5):S56–S62
Olsen J, Peroski M, Kiczek M, Grignol G, Merchenthaler I, Dudas B (2014) Intimate associations between the endogenous opiate systems and the growth hormone-releasing hormone system in the human hypothalamus. Neuroscience 258:238–245
Pralong FP (2010) Insulin and NPY pathways and the control of GNRH function and puberty onset. Mol Cell Endocrinol 324:82–86
Quennell JH, Mulligan AC, Tups A et al (2009) Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 150:2805–2812
Reichmann F, Holzer P (2016) Neuropeptide Y: a stressful review. Neuropeptides 55:99–109
Roa J, Herbison AE (2012) Direct regulation of GnRH neuron excitability by arcuate nucleus POMC and NPY neuron neuropeptides in female mice. Endocrinology 153:5587–5599
Roa J, Tena-Sempere M (2010) Energy balance and puberty onset: emerging role of central mTOR signaling. Trends Endocrinol Metab 21:519–528
Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM (2004) Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in Ob/Ob mice. Neuron 41(5):P711–P722
Sahu A, Crowley WR, Kalra SP (1990) An opioid-neuropeptide-Y transmission line to luteinizing hormone (LH)-releasing hormone neurons: a role in the induction of LH surge. Endocrinology 126:876–883
Saper CB (1990) Hypothalamus. In: Paxinos G (ed) The human nervous system. Academic Press, San Diego, pp 389–413
Skrapits K, Borsay BE, Herczeg L et al (2014) Colocalization of cocaine-and amphetamine-regulated transcript with kisspeptin and neurokinin N in the human infundibular region. PLoS One 9:E103977
Stincic TL, Grachev P, Bosch MA, Ronnekleiv OK, Kelly MJ (2018) Estradiol drives the anorexigenic activity of proopiomelanocortin neurons in female mice. Eneuro. https://doi.org/10.1523/ENEURO.0103-18.2018
Swaab DF (2003) The human hypothalamus: basic and clinical aspects. Part 1: nuclei of the human hypothalamus. Elsevier
Thind KK, Goldsmith PC (1988) Infundibular gonadotropin-releasing hormone neurons are inhibited by direct opioid and autoregulatory synapses in Juvenile monkeys. Neuroendocrinology 47:203–216
True C, Takahashi D, Kirigiti M, Lidsley SR, Moctezuma C, Arik A, Smith MS, Kievit P, Grove KL (2017) Arcuate nucleus neuropeptide coexpression and connections to gonadotropin-releasing hormone neurons in the female rhesus macaque. J Neuroendocrinol. https://doi.org/10.1111/Jne.12491
Veening JG, Barendregt HP (2015) The effects of beta-endorphin: state change modification. Fluids Barriers CNS 12:3
Wade GN, Jones JE (2004) Neuroendocrinology of nutritional infertility. Am J Physiol Regul Integr Comp Physiol 287:R1277–R1296
Yang Y, Atasoy D, Su HH, Sternson SM (2011) Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 146:992–1003
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human and animal participants
The brains utilized in these studies were harvested at 12-h post-mortem period in accordance with the regulations of the Institutional Review Board of Lake Erie College of Osteopathic Medicine (LECOM). See materials and methods.
Informed consent
not applicable
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dudas, B., Merchenthaler, I. Β-endorphin-immunoreactive perikarya appear to receive innervation from NPY-immunoreactive fiber varicosities in the human hypothalamus. Brain Struct Funct 227, 821–828 (2022). https://doi.org/10.1007/s00429-021-02416-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02416-3