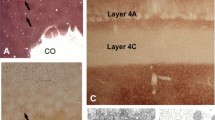
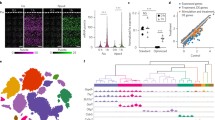
Abstract
Gene expression gradients radiating from regions of primary sensory cortices have recently been described and are thought to underlie the large-scale organization of the human cerebral cortex. However, the role of transcription in the functional layout of a single region within the adult brain has yet to be clarified, likely owing to the difficulty of identifying a brain region anatomically consistent enough across individuals with dense enough tissue sampling. Overcoming these hurdles in human primary visual cortex (V1), we show a relationship between differential gene expression and the cortical layout of eccentricity in human V1. Interestingly, these genes are unique from those previously identified that contribute to the positioning of cortical areas in the visual processing hierarchy. Enrichment analyses show that a subset of the identified genes encode for structures related to inhibitory interneurons, ion channels, as well as cellular projections, and are expressed more in foveal compared to peripheral portions of human V1. These findings predict that tissue density should be higher in foveal compared to peripheral V1. Using a histological pipeline, we validate this prediction using Nissl-stained sections of postmortem occipital cortex. We discuss these findings relative to previous studies in non-human primates, as well as in the context of an organizational pattern in which the adult human brain employs transcription gradients at multiple spatial scales: across the cerebral cortex, across areas within processing hierarchies, and within single cortical areas.



Similar content being viewed by others
Data availability
All analyzed data were curated from the following open datasets. Allen Human Brain Atlas (AHBA) Microarray Data: http://human.brain-map.org. Neuropythy atlas of human visual cortex: https://github.com/noahbenson/neuropythy. BrainSpan histological stains: http://www.brainspan.org/static/atlas.
Code availability
Code related to processing of AHBA transcription data can be found on author ZZ’s GitHub at: https://github.com/zhenzonglei/matni, and code for the beta version of BrainWalker can be found at: https://github.com/gomezj/geneccentricity.
References
Alvarez I, Finlayson NJ, Ei S, de Haas B, Greenwood JA, Schwarzkopf DS (2021) Heritable functional architecture in human visual cortex. NeuroImage 239:118286.
Amunts K, Zilles K (2015) Architectonic mapping of the human brain beyond brodmann. Neuron 88:1086–1107
Arcaro MJ, Kastner S (2015) Topographic organization of areas V3 and V4 and its relation to supra-areal organization of the primate visual system. Vis Neurosci 32:E014
Arnatkeviciute A, Fulcher BD, Fornito A (2019) A practical guide to linking brain-wide gene expression and neuroimaging data. Neuroimage 189:353–367
Azzopardi P, Jones KE, Cowey A (1999) Uneven mapping of magnocellular and parvocellular projections from the lateral geniculate nucleus to the striate cortex in the macaque monkey. Vision Res 39:2179–2189
Balaram P, Kaas JH (2014) Towards a unified scheme of cortical lamination for primary visual cortex across primates: insights from NeuN and VGLUT2 immunoreactivity. Front Neuroanat 8:81
Barone P, Debay C, Berland M, Kennedy H (1996) Role of directed growth and target selection in the formation of cortical pathways: prenatal development of the projection of area V2 to area V4 in the monkey. J Comp Neurol 374:1–20
Batardiere A, Barone P, Dehay C, Kennedy H (1998) Area-specific laminar distribution of cortical feedback neurons projecting to cat area 17: quantitative analysis in the adult and during ontogeny. J Comp Neurol 396:493–510
Benson NC, Winawer J (2018) Bayesian analysis of retinotopic maps. Elife 7:e40224
Benson NC et al (2021) Variability of the surface area of the V1, V2, and V3 maps in a large sample of human observers. bioRxiv 109:816
Burt JB et al (2018) Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci 93:165
Chen J, Bardes EE, Aronow BJ, Jegga AG (2009) ToppGene Suite for gene list enrichment analysis and candidate gene prioritization. Nucleic Acids Res 37:W305-311
Collins CE, Airey DC, Young NA, Leitch DB, Kaas JH (2010) Neuron densities vary across and within cortical areas in primates. Proc Natl Acad Sci U S A 107:15927–15932
Collins CE, Turner EC, Sawyer EK, Reed JL, Young NA, Flaherty DK, Kaas JH (2016) Cortical cell and neuron density estimates in one chimpanzee hemisphere. Proc Nat Acad Sci 113(3):740–745
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis I. Segmentation and surface reconstruction. Neuroimage 9:179–194
Eickhoff SB, Rottschy C, Zilles K (2007) Laminar distribution and co-distribution of neurotransmitter receptors in early human visual cortex. Brain Struct Funct 212:255–267
Eickhoff SB, Yeo BTT, Genon S (2018) Imaging-based parcellations of the human brain. Nat Rev Neurosci 19:672–686
Fischl MI, Sereno AM, Dale, (1999) Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 9:195–207
Fornito A, Arnatkeviciute A, Fulcher BD (2019) Bridging the gap between connectome and transcriptome. Trends Cogn Sci 23:34–50
Fulcher BD (2019) Discovering conserved properties of brain organization through multimodal integration and interspecies comparison. J Exp Neurosci 13:1179069519862047
Gomez J, Natu V, Jeska B, Barnett M, Grill-Spector K (2018) Development differentially sculpts receptive fields across early and high-level human visual cortex. Nat Commun 9:788
Gomez J et al (2019) Development of population receptive fields in the lateral visual stream improves spatial coding amid stable structural-functional coupling. Neuroimage 188:59–69
Gomez J, Zhen Z, Weiner KS (2019) Human visual cortex is organized along two genetically opposed hierarchical gradients with unique developmental and evolutionary origins. PLoS Biol. 17:e3000362
Hawrylycz MJ et al (2012) An anatomically comprehensive atlas of the adult human brain transcriptome. Natures 489:391–399
Honig LS, Herrmann K, Shatz CJ (1996) Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex 6:794–806
Horiguchi H, Nakadomari S, Misaki M, Wandell BA (2009) Two temporal channels in human V1 identified using fMRI. Neuroimage 47:273–280
Huntenburg JM, Bazin PL, Margulies DS (2018) Large-scale gradients in human cortical organization. Trends Cogn Sci 22:21–31
Jones AR, Overly CC, Sunkin SM (2009) The Allen Brain Atlas: 5 years and beyond. Nat Rev Neurosci 10:821–828
Kennedy H, Dehay C, Bullier J (1986) Organization of the callosal connections of visual areas V1 and V2 in the macaque monkey. J Comp Neurol 247:398–415
Khrameeva E et al (2020) Single-cell-resolution transcriptome map of human, chimpanzee, bonobo, and macaque brains. Genome Res 30:776–789
Kohonen T (1982) Self-organized formation of topologically correct feature maps. Biol Cybern 43:59–69
Krienen FM, Yeo BT, Ge T, Buckner RL, Sherwood CC (2016) Transcriptional profiles of supragranular-enriched genes associate with corticocortical network architecture in the human brain. Proc Natl Acad Sci U S A 113:E469-478
Perkel DJ, Bullier J, Kennedy H (1986) Topography of the afferent connectivity of area 17 in the macaque monkey: a double-labelling study. J Comp Neurol 253:374–402
Rakic P, Goldman-Rakic PS, Gallager D (1988) Quantitative autoradiography of major neurotransmitter receptors in the monkey striate and extrastriate cortex. J Neurosci 8:3670–3690
Rosa MG (2002) Visual maps in the adult primate cerebral cortex: some implications for brain development and evolution. Braz J Med Biol Res 35:1485–1498
Rosa MG, Tweedale R (2005) Brain maps, great and small: lessons from comparative studies of primate visual cortical organization. Philos Trans R Soc Lond B Biol Sci 360:665–691
Schleicher AZ, Zilles K, Wree A (1986) A quantitative approach to cytoarchitectonics: software and hardware aspects of a system for the evaluation and analysis of structural inhomogeneities in nervous tissue. J Neurosci Methods 18:221–235
Schwarzkopf DS, Song C, Rees G (2011) The surface area of human V1 predicts the subjective experience of object size. Nat Neurosci 14:28–30
Seidlitz J et al (2020) Transcriptomic and cellular decoding of regional brain vulnerability to neurogenetic disorders. Nat Commun 11:3358
Sestan N, Rakic P, Donoghue MJ (2001) Independent parcellation of the embryonic visual cortex and thalamus revealed by combinatorial Eph/ephrin gene expression. Curr Biol 11:39–43
Smart IHM, McSherry GM (1986) Gyrus formation in the cerebral cortex of the ferret. Description of the internal histological changes. J Anat 147:27–43
Stigliani A, Jeska B, K, (2017) Grill-spector, encoding model of temporal processing in human visual cortex. Proc Nat Acad Sci 41:788
Syken J, Grandpre T, Kanold PO, Shatz CJ (2006) PirB restricts ocular-dominance plasticity in visual cortex. Science 313:1795–1800
Takahata T, Shukla R, Yamamori T, Kaas JH (2012) Differential expression patterns of striate cortex-enriched genes among Old World, New World, and prosimian primates. Cereb Cortex 22(10):2313–2321
Valk SL et al (2020) Shaping brain structure: genetic and phylogenetic axes of macroscale organization of cortical thickness. Sci Adv 6:eabb3417
Wandell BA, Winawer J (2011) Imaging retinotopic maps in the human brain. Vision Res 51:718–737
Wandell BA, Winawer J (2015) Computational neuroimaging and population receptive fields. Trends Cogn Sci 19:349–357
Watakabe A (2009) Comparative molecular neuroanatomy of mammalian neocortex: what can gene expression tell us about areas and layers? Dev Growth Differ 51:343–354
Watakabe A et al (2007) Comparative analysis of layer-specific genes in Mammalian neocortex. Cereb Cortex 17:1918–1933
Wei Y et al (2019) Genetic mapping and evolutionary analysis of human-expanded cognitive networks. Nat Commun 10:4839
Yamamori T, Rockland KS (2006) Neocortical areas, layers, connections, and gene expression. Neurosci Res 55:11–27
Zilles K, Palomero-Gallagher N (2017) Multiple transmitter receptors in regions and layers of the human cerebral cortex. Front Neuroanat 11:78
Funding
This work was supported by (1) start-up funds provided by the University of California, Berkeley and the Helen Wills Neuroscience Institute to KSW; (2) start-up funds provided by Princeton Neuroscience Institute to JG; and (3) The National Natural Science Foundation of China 31771251 awarded to ZZ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest, financial or otherwise.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gomez, J., Zhen, Z. & Weiner, K.S. The relationship between transcription and eccentricity in human V1. Brain Struct Funct 226, 2807–2818 (2021). https://doi.org/10.1007/s00429-021-02387-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02387-5