Abstract
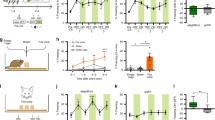
A few studies have evaluated the behavioral roles of the periaqueductal gray (PAG) in animals facing ethologically relevant threats. Exposure to a live cat induces striking activation in the rostrodorsal and caudal ventral PAG. In the present investigation, we first showed that cytotoxic lesions of the rostrodorsal and caudal ventral PAG had similar effects on innate fear responses during cat exposure, practically abolishing freezing and increasing risk assessment responses. Conversely, rostrodorsal PAG lesions but not caudal ventral lesions disrupted learned contextual fear responses to cat exposure. Next, we examined how muscimol inactivation of the rostrodorsal PAG at different times (i.e., during, immediately after and 20 min after cat exposure) influences learned contextual fear responses, and we found that inactivation of the rostrodorsal PAG during or immediately after cat exposure but not 20 min later impaired contextual fear learning. Thus, suggesting that the rostrodorsal PAG is involved in the acquisition, but not the consolidation, of contextual fear memory to predatory threat. Notably, the dosolateral PAG contains a distinct population of neurons containing the neuronal nitric oxide synthase (nNOS) enzyme, and in the last experiment, we investigated how nitric oxide released in rostrodorsal PAG influences contextual fear memory processing. Accordingly, injection of a selective nNOS inhibitor into the rostrodorsal PAG immediately after cat exposure disrupted learned contextual responses. Overall, the present findings suggest that the acquisition of contextual fear learning is influenced by an optimum level of dorsal PAG activation, which extends from during to shortly after predator exposure and depends on local NO release.









Similar content being viewed by others
References
Aguiar DC, Guimarães FS (2009) Blockade of NMDA receptors and nitric oxide synthesis in the dorsolateral periaqueductal gray attenuates behavioral and cellular responses of rats exposed to a live predator. J Neurosci Res 87:2418–2429
Assareh N, Sarrami M, Carrive P, McNally GP (2016) The organization of defensive behavior elicited by optogenetic excitation of rat lateral or ventrolateral periaqueductal gray. Behav Neurosci 130:406–414
Back FP, Carobrez AP (2018) Periaqueductal gray glutamatergic, cannabinoid and, vanilloid receptor interplay in defensive behavior and aversive memory formation. Neuropharmacology 135:399–411
Bandler R, Keay KA (1996) Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res 107:285–300
Bandler R, Shipley MT (1994) Columnar organization of the midbrain periaqueductal gray: modules for emotional expression. Trends Neurosci 17:379–389
Bindi RP, Baldo MVC, Canteras NS (2018) Roles of the anterior basolateral amygdalar nucleus during exposure to a live predator and to a predator-associated context. Behav Brain Res 342:51–56
Bittencourt AS, Carobrez AP, Zamprogno LP, Tufik S, Schenberg LC (2004) Organization of single components of defensive behaviors within distinct columns of periaqueductal gray matter of the rat: role of N-methyl-d-aspartic acid glutamate receptors. Neuroscience 125:71–89
Blanchard RJ, Blanchard DC, Hori K (1989) Ethoexperimental approaches to the study of defensive behavior. In: Blanchard RJ, Brain PF, Blanchard DC, Parmigiani S (eds) Ethoexperimental approaches to the study of behavior. Academic Publishers, Dordrecht, pp 114–136
Blanchard DC, Li CI, Hubbard D, Markham CM, Yang M, Takahashi LK, Blanchard RJ (2003) Dorsal premammillary nucleus differentially modulates defensive behaviors induced by different threat stimuli in rats. Neurosci Lett 345:145–148
Blanchard DC, Griebel G, Pobbe R, Blanchard RJ (2011) Risk assessment as an evolved threat detection and analysis process. Neurosci Biobehav Rev 35:991–998
Carrive P (1993) The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res 58:27–47
Cezário AF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS (2008) Hypothalamic sites responding to predator threats—the role of the dorsal premammillary nucleus in unconditioned and conditioned antipredatory defensive behavior. Eur J Neurosci 28:1003–1015
Chiavegatto S, Scavone C, Canteras NS (1998) Nitric oxide synthase activity in the dorsal periaqueductal gray of rats expressing innate fear responses. Neuroreport 9:571 576
Comoli E, Ribeiro-Barbosa ER, Canteras NS (2000) Afferent connections of the dorsal premammillary nucleus. J Comp Neurol 423:83–98
Comoli E, Ribeiro-Barbosa ER, Canteras NS (2003) Predatory hunting and exposure to a live predator induce opposite patterns of Fos immunoreactivity in the PAG. Behav Brain Res 138:17–28
De Oca BM, DeCola JP, Maren S, Fanselow MS (1998) Distinct regions of the periaqueductal gray are involved in the acquisition and expression of defensive responses. J Neurosci 18:3426–3432
de Lima MA, Baldo MV, Canteras NS (2017) A role for the anteromedial thalamic nucleus in the acquisition of contextual fear memory to predatory threats. Brain Struct Funct 222:113–129
de Lima MA, Baldo MV, Canteras NS (2018) Revealing a cortical circuit responsive to predatory threats and mediating contextual fear memory. Cereb Cortex. https://doi.org/10.1093/cercor/bhy173
Deng H, Xiao X, Wang Z (2016) Periaqueductal gray neuronal activities underlie different aspects of defensive behaviors. J Neurosci 36:7580–7588
Di Scala G, Sandner G (1989) Conditioned place aversion produced by microinjections of semicarbazide into the periaqueductal gray of the rat. Brain Res 483:91–97
Di Scala G, Mana MJ, Jacobs WJ, Phillips AG (1987) Evidence of Pavlovian conditioned fear following electrical stimulation of the periaqueductal grey in the rat. Physiol Behav 40:55–63
Edeline JM, Hars B, Hennevin E, Cotillon N (2002) Muscimol diffusion after intracerebral microinjections: a reevaluation based on electrophysiological and autoradiographic quantifications. Neurobiol Learn Mem 78:100–124
Fanselow MS (1991) The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: Depaulis A, Bandler R (eds) The midbrain periaqueductal gray matter: functional, anatomical and neurochemical organization. Plenum Press, New York, pp 151–173
Furlong TM, Cole S, Hamlin AS, McNally GP (2010) The role of prefrontal cortex in predictive fear learning. Behav Neurosci 124:574–586
Goto M, Canteras NS, Burns G, Swanson LW (2005) Projections from the subfornical region of the lateral hypothalamic area. J Comp Neurol 493:412–438
Gray JA, McNaughton N (2000) The neuropsychology of anxiety, 2nd edn. University Press, Oxford
Guimarães FS, Beijamini V, Moreira FA, Aguiar DC, de Lucca AC (2005) Role of nitric oxide in brain regions related to defensive reactions. Neurosci Biobehav Rev 29:1313–1322
Johansen JP, Tarpley JW, LeDoux JE, Blair HT (2010) Neural substrates for expectation-modulated fear learning in the amygdala and periaqueductal gray. Nat Neurosci 13:979–998
Kim EJ, Horovitz O, Pellman BA, Tan LM, Li Q, Richter-Levin G, Kim JJ (2013) Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proc Natl Acad Sci USA 110:14795–14800
Kincheski GC, Mota-Ortiz SR, Pavesi E, Canteras NS, Carobrez AP (2012) The dorsolateral periaqueductal gray and its role in mediating fear learning to life threatening events. PLoS One 7:e50361
Koutsikou S, Crook JJ, Earl EV, Leith JL, Watson TC, Lumb BM, Apps R (2014) Neural substrates underlying fear-evoked freezing: the periaqueductal grey-cerebellar link. J Physiol 592:2197–2213
Koutsikou S, Watson TC, Crook JJ, Leith JL, Lawrenson CL, Apps R, Lumb BM (2015) The periaqueductal gray orchestrates sensory and motor circuits at multiple levels of the neuraxis. J Neurosci 35:14132–14147
LeDoux JE, Iwata J, Cicchetti P, Reis DJ (1988) Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 8:2517–2529
Linke R, Braune G, Schwegler H (2000) Differential projection of the posterior paralaminar thalamic nuclei to the amygdaloid complex in the rat. Exp Brain Res 134:520–532
Martin JH (1991) Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett 127:160–164
Martinez RC, Carvalho-Netto EF, Ribeiro-Barbosa ER, Baldo MV, Canteras NS (2011) Amygdalar roles during exposure to a live predator and to a predator-associated context. Neuroscience 172:314–328
Motta SC, Carobrez AP, Canteras NS (2017) The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neurosci Biobehav Rev 76:39–47
Onstott D, Mayer B, Beitz AJ (1993) Nitric oxide synthase immunoreactive neurons anatomically define a longitudinal dorsolateral column within the midbrain periaqueductal gray of the rat: analysis using laser confocal microscopy. Brain Res 610:317–324
Ribeiro-Barbosa ER, Canteras NS, Cezário AF, Blanchard RJ, Blanchard DC (2005) An alternative experimental procedure for studying predator-related defensive responses. Neurosci Biobehav Rev 29:1255–1263
Risold PY, Canteras NS, Swanson LW (1994) Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348:1–40
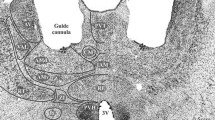
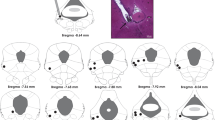
Rufino RA (2015) Investigation of periaqueductal gray (PAG) in the organization of defensive antipredatory behavior. Master Thesis. Institute of Biomedical Sciences, University of São Paulo
Souza RR, Carobrez AP (2016) Acquisition and expression of fear memories are distinctly modulated along the dorsolateral periaqueductal gray axis of rats exposed to predator odor. Behav Brain Res 315:160–167
Swanson LW (2004) Brain maps structure of the rat brain, 3rd edn. Elsevier, Amesterdam
Tabachnick BG, Fidell LS (2007) Using multivariate statistics, 5th edn. Pearson Education Inc, Boston
Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SB, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Lüthi A (2016) Midbrain circuits for defensive behaviour. Nature 534:206–221
van Groen T, Wyss JM (1992) Projections from the laterodorsal nucleus of the thalamus to the limbic and visual cortices in the rat. J Comp Neurol 324:427–448
Vertes RP, Hoover WB, Do Valle AC, Sherman A, Rodriguez JJ (2006) Efferent projections of reuniens and rhomboid nuclei of the thalamus in the rat. J Comp Neurol 499:768–796
Vianna DM, Landeira-Fernandez J, Brandão ML (2001) Dorsolateral and ventral regions of the periaqueductal gray matter are involved in distinct types of fear. Neurosci Biobehav Rev 25:711–719
Vincent SR, Kimura H (1992) Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46:755–784
Walker P, Carrive P (2003) Role of ventrolateral periaqueductal gray neurons in the behavioral and cardiovascular responses to contextual conditioned fear and poststress recovery. Neuroscience 116:897–912
Acknowledgements
This research was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)—Research Grants #2014/05432-9 (awarded to N.S.C) and #2009/53390-5 (awarded to S.R.M.O.). R.A.R. and M.A.X.L. were supported by FAPESP fellowships (R.A.R. #2014/02540-5/M.A.X.L. #2016/10389-0).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Research involving human participants and/or animals
Experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, 1996). All experimental procedures had been previously approved by the Committee on The Care and Use of Laboratory Animals of the Institute of Biomedical Sciences, University of São Paulo, Brazil (Protocol number 085/2012). In the present study, the experiments were planned to minimize the number of animals used and their suffering. In addition, all surgical procedures were performed under deep anesthesia, and analgesic and antibiotic medications were given postoperatively.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Andrade Rufino, R., Mota-Ortiz, S.R., De Lima, M.A.X. et al. The rostrodorsal periaqueductal gray influences both innate fear responses and acquisition of fear memory in animals exposed to a live predator. Brain Struct Funct 224, 1537–1551 (2019). https://doi.org/10.1007/s00429-019-01852-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-019-01852-6