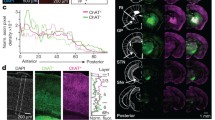
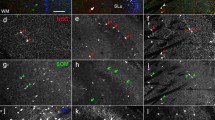
Abstract
Dopamine is critical for the normal functioning of the basal ganglia, modulating both input and output nuclei of this system. The distribution and function of each of the five dopamine receptor subtypes have been studied extensively in the striatum. However, the role of extrastriatal dopamine receptors in basal ganglia information processing is less clear. Here, we studied the anatomical distribution of dopamine receptors in one of the output nuclei of the rodent basal ganglia, the entopeduncular nucleus (EP). The presence of all dopamine receptor subtypes was verified in the EP using immunostaining. We detected co-localization of dopamine receptors with VGAT, which suggests presynaptic expression on GABAergic terminals. D1R and D2R were strongly colocalized with VGAT, whereas DR3-5 showed only sparse co-localization. We further labeled striatal or pallidal neurons with GFP and showed that only D1 receptors were co-localized with striatal terminals, while only D2R and D3R were co-localized with pallidal terminals. Dopamine receptors were also strongly co-localized with MAP2, indicating postsynaptic expression. Overall, these findings suggest that the dopaminergic system modulates activity in the EP both directly via postsynaptic receptors, and indirectly via GABAergic synapses stemming from the direct and indirect pathways.








Similar content being viewed by others
References
Aceves J, Floran B, Sierra A, Mariscal S (1995) D1 receptor mediated modulation of the release of gamma-aminobutyric acid by endogenous dopamine in the basal ganglia of the rat. Prog Neuropsychopharmacol Biol Psychiatry 19:727–739
Alexander G (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Alexander GE, Crutcher MD (1990) Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 13:266–271
Bamford NS, Zhang H, Schmitz Y et al (2004) Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron 42:653–663
Beckstead RM, Domesick VB, Nauta WJ (1979) Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res 175:191–217
Beninger RJ (1983) The role of dopamine in locomotor activity and learning. Brain Res 287:173–196
Bolam JP, Smith Y (1992) The striatum and the globus pallidus send convergent synaptic inputs onto single cells in the entopeduncular nucleus of the rat: a double anterograde labelling study combined with postembedding immunocytochemistry for GABA. J Comp Neurol 321:456–476
Bolam JP, Hanley JJ, Booth PA, Bevan MD (2000) Synaptic organisation of the basal ganglia. J Anat 196(Pt 4):527–542
Boyson SJ, McGonigle P, Molinoff PB (1986) Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci 6:3177–3188
Caille I, Dumartin B, Bloch B (1996) Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res 730:17–31
Ciliax BJ, Nash N, Heilman C et al (2000) Dopamine D 5 receptor immunolocalization in rat and monkey brain. Synapse 37:125–145
Clarke NP, Bolam JP, Bevan MD (1996) Glutamate-enriched inputs from the mesopontine tegmentum to the entopeduncular nucleus in the rat. Eur J Neurosci 8:1363–1376
Dawson TM, Gehlert DR, McCabe RT et al (1986) D-1 dopamine receptors in the rat brain: a quantitative autoradiographic analysis. J Neurosci 6:2352–2365
Deniau JM, Hammond C, Chevalier G, Feger J (1978) Evidence for branched subthalamic nucleus projections to substantia nigra, entopeduncular nucleus and globus pallidus. Neurosci Lett 9:117–121
Floran B, Aceves J, Sierra A, Martinez-Fong D (1990) Activation of D1 dopamine receptors stimulates the release of GABA in the basal ganglia of the rat. Neurosci Lett 116:136–140
Fuchs H, Hauber W (2004) Dopaminergic innervation of the rat globus pallidus characterized by microdialysis and immunohistochemistry. Exp Brain Res 154:66–75
Gauthier J, Parent M, Levesque M, Parent A (1999) The axonal arborization of single nigrostriatal neurons in rats. Brain Res 834:228–232
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466
Kliem MA, Maidment NT, Ackerson LC et al (2007) Activation of nigral and pallidal dopamine D1-like receptors modulates basal ganglia outflow in monkeys. J Neurophysiol 98:1489–1500
Kliem MA, Pare JF, Khan ZU et al (2010) Ultrastructural localization and function of dopamine D1-like receptors in the substantia nigra pars reticulata and the internal segment of the globus pallidus of parkinsonian monkeys. Eur J Neurosci 31:836–851
Kumar R, Lozano AM, Montgomery E, Lang AE (1998) Pallidotomy and deep brain stimulation of the pallidum and subthalamic nucleus in advanced Parkinson’s disease. Mov Disord 13 Suppl 1:73–82
Lavian H, Almog M, Madar R et al (2017) Dopaminergic modulation of synaptic integration and firing patterns in the rat entopenduncular nucleus. J Neurosci 37(30):7177–7187
Lindvall O, Bjorklund A, Moore RY, Stenevi U (1974) Mesencephalic dopamine neurons projecting to neocortex. Brain Res 81:325–331
Maia TV, Frank MJ (2011) From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci 14:154–162
Mansour A, Meador-Woodruff JH, Zhou Q et al (1992) A comparison of D1 receptor binding and mRNA in rat brain using receptor autoradiographic and in situ hybridization techniques. Neuroscience 46:959–971
Mink JW (1996) The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol 50:381–425
Mitkovski M, Padovan-Neto FE, Raisman-Vozari R et al (2012) Investigations into potential extrasynaptic communication between the dopaminergic and nitrergic systems. Front Physiol 3:372
Nagy JI, Carter DA, Fibiger HC (1978) Anterior striatal projections to the globus pallidus, entopeduncular nucleus and substantia nigra in the rat: the GABA connection. Brain Res 158:15–29
Nakahara H, Itoh H, Kawagoe R et al (2004) Dopamine neurons can represent context-dependent prediction error. Neuron 41:269–280
Nambu A (2007) Globus pallidus internal segment. Prog Brain Res 160:135–150
Neve KA, Seamans JK, Trantham-Davidson H (2004) Dopamine receptor signaling. J Recept Signal Transduct Res 24:165–205
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates: hard cover edition. Elsevier Science, Amsterdam
Pickel VM, Beckley SC, Joh TH, Reis DJ (1981) Ultrastructural immunocytochemical localization of tyrosine hydroxylase in the neostriatum. Brain Res 225:373–385
Rivera A, Trias S, Penafiel A et al (2003) Expression of D4 dopamine receptors in striatonigral and striatopallidal neurons in the rat striatum. Brain Res 989:35–41
Ruskin DN, Bergstrom DA, Walters JR (2002) Nigrostriatal lesion and dopamine agonists affect firing patterns of rodent entopeduncular nucleus neurons. J Neurophysiol 88:487–496
Schrock LE, Mink JW, Woods DW et al (2014) Tourette syndrome deep brain stimulation: a review and updated recommendations. Mov Disord 30:448–471
Schultz W, Apicella P, Scarnati E, Ljungberg T (1992) Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci 12:4595–4610
Siegfried J, Lippitz B (1994) Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery 35:1126–1130
Smith Y, Lavoie B, Dumas J, Parent A (1989) Evidence for a distinct nigropallidal dopaminergic projection in the squirrel monkey. Brain Res 482:381–386
Stojanovic T, Orlova M, Sialana FJ et al (2017) Validation of dopamine receptor DRD1 and DRD2 antibodies using receptor deficient mice. Amino Acids 49:1101–1109
Straub C, Tritsch NX, Hagan NA et al (2014) Multiphasic modulation of cholinergic interneurons by nigrostriatal afferents. J Neurosci 34:8557–8569
Surmeier DJ, Eberwine J, Wilson CJ et al (1992) Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA 89:10178–10182
Tritsch NX, Sabatini BL (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76:33–50
Wang Q, Ji T, Zheng LF et al (2012) Cellular localization of dopamine receptors in the gastric mucosa of rats. Biochem Biophys Res Commun 417:197–203
Watabe-Uchida M, Zhu L, Ogawa SK et al (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873
Wickens JR, Horvitz JC, Costa RM, Killcross S (2007) Dopaminergic mechanisms in actions and habits. J Neurosci 27:8181–8183
Yung KK, Bolam JP, Smith AD et al (1995) Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience 65:709–730
Acknowledgements
The authors would like to thank Dr. Avi Jacob for his excellent assistance with confocal imaging and Ziv Almog for his excellent assistance with graphic design.
Funding
This work was supported by a Grant from the Israel Science Foundation—Heritage Biomedical Science Partnership program of the Israel Science Foundation to AK and IBG (#138/15), and the Paul Feder foundation for neurodegenerative disorder research Grant to EO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
All experimental procedures were approved and supervised by the Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Bar-Ilan University Guidelines for the Use and Care of Laboratory Animals in Research. This study was approved by the Israel National Committee for Experiments in Laboratory Animals at the Ministry of Health. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Lavian, H., Loewenstern, Y., Madar, R. et al. Dopamine receptors in the rat entopeduncular nucleus. Brain Struct Funct 223, 2673–2684 (2018). https://doi.org/10.1007/s00429-018-1657-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1657-6