Abstract
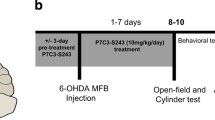
Dopamine loss and motor deficits in Parkinson’s disease typically commence unilaterally and remain asymmetric for many years, raising the possibility that endogenous defenses slow the cross-hemispheric transmission of pathology. It is well-established that the biological response to subtoxic stress prepares cells to survive subsequent toxic challenges, a phenomenon known as preconditioning, tolerance, or stress adaptation. Here we demonstrate that unilateral striatal infusions of the oxidative toxicant 6-hydroxydopamine (6-OHDA) precondition the contralateral nigrostriatal pathway against the toxicity of a second 6-OHDA infusion in the opposite hemisphere. 6-OHDA-induced loss of dopaminergic terminals in the contralateral striatum was ablated by cross-hemispheric preconditioning, as shown by two independent markers of the dopaminergic phenotype, each measured by two blinded observers. Similarly, loss of dopaminergic somata in the contralateral substantia nigra was also abolished, according to two blinded measurements. Motor asymmetries in floor landings, forelimb contacts with a wall, and spontaneous turning behavior were consistent with these histological observations. Unilateral 6-OHDA infusions increased phosphorylation of the kinase ERK2 and expression of the antioxidant enzyme CuZn superoxide dismutase in both striata, consistent with our previous mechanistic work showing that these two proteins mediate preconditioning in dopaminergic cells. These findings support the existence of cross-hemispheric preconditioning in Parkinson’s disease and suggest that dopaminergic neurons mount impressive natural defenses, despite their reputation as being vulnerable to oxidative injury. If these results generalize to humans, Parkinson’s pathology may progress slowly and asymmetrically because exposure to a disease-precipitating insult induces bilateral upregulation of endogenous defenses and elicits cross-hemispheric preconditioning.






Similar content being viewed by others
Abbreviations
- 6-OHDA:
-
6-Hydroxydopamine
- GAP43:
-
Growth-associated protein 43
- PBS:
-
Phosphate-buffered saline
- DAT:
-
Dopamine transporter
- CuZnSOD:
-
CuZn superoxide dismutase
- MnSOD:
-
Manganese superoxide dismutase
- TBS:
-
Tris-buffered saline
- TH:
-
Tyrosine hydroxylase
References
Allen NJ (2014) Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol 30:439–463. https://doi.org/10.1146/annurev-cellbio-100913-013053
Annett LE, Rogers DC, Hernandez TD, Dunnett SB (1992) Behavioural analysis of unilateral monoamine depletion in the marmoset. Brain 115(Pt 3):825–856
Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ (1998) Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke 29(9):1937–1950 (discussion 1950–1931)
Barrett MJ, Wylie SA, Harrison MB, Wooten GF (2011) Handedness and motor symptom asymmetry in Parkinson’s disease. J Neurol Neurosurg Psychiatry 82(10):1122–1124. https://doi.org/10.1136/jnnp.2010.209783
Batassini C, Broetto N, Tortorelli LS, Borsoi M, Zanotto C, Galland F, Souza TM, Leite MC, Goncalves CA (2015) Striatal injury with 6-OHDA transiently increases cerebrospinal GFAP and S100B. Neural Plast 2015:387028. https://doi.org/10.1155/2015/387028
Bezard E, Gross CE, Brotchie JM (2003) Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci 26(4):215–221. https://doi.org/10.1016/S0166-2236(03)00038-9
Blesa J, Juri C, Garcia-Cabezas MA, Adanez R, Sanchez-Gonzalez MA, Cavada C, Obeso JA (2011) Inter-hemispheric asymmetry of nigrostriatal dopaminergic lesion: a possible compensatory mechanism in Parkinson’s disease. Front Syst Neurosci 5:92. https://doi.org/10.3389/fnsys.2011.00092
Boger HA, Granholm AC, McGinty JF, Middaugh LD (2010) A dual-hit animal model for age-related parkinsonism. Prog Neurobiol 90(2):217–229. https://doi.org/10.1016/j.pneurobio.2009.10.013
Boix J, Padel T, Paul G (2015) A partial lesion model of Parkinson’s disease in mice—characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav Brain Res 284:196–206. https://doi.org/10.1016/j.bbr.2015.01.053
Boonstra TA, Schouten AC, van Vugt JP, Bloem BR, van der Kooij H (2014) Parkinson’s disease patients compensate for balance control asymmetry. J Neurophysiol 112(12):3227–3239. https://doi.org/10.1152/jn.00813.2013
Boulet S, Mounayar S, Poupard A, Bertrand A, Jan C, Pessiglione M, Hirsch EC, Feuerstein C, Francois C, Feger J, Savasta M, Tremblay L (2008) Behavioral recovery in MPTP-treated monkeys: neurochemical mechanisms studied by intrastriatal microdialysis. J Neurosci 28(38):9575–9584. https://doi.org/10.1523/JNEUROSCI.3465-08.2008
Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AC (1995) Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol 355(4):479–489
Brotchie J, Fitzer-Attas C (2009) Mechanisms compensating for dopamine loss in early Parkinson disease. Neurology 72(7 Suppl):S32–S38. https://doi.org/10.1212/WNL.0b013e318198e0e9
Brown P, Gerfen CR (2006) Plasticity within striatal direct pathway neurons after neonatal dopamine depletion is mediated through a novel functional coupling of serotonin 5-HT2 receptors to the ERK 1/2 map kinase pathway. J Comp Neurol 498(3):415–430. https://doi.org/10.1002/cne.21034
Busse K, Heilmann R, Kleinschmidt S, Abu-Mugheisib M, Hoppner J, Wunderlich C, Gemende I, Kaulitz L, Wolters A, Benecke R, Walter U (2012) Value of combined midbrain sonography, olfactory and motor function assessment in the differential diagnosis of early Parkinson’s disease. J Neurol Neurosurg Psychiatry 83(4):441–447. https://doi.org/10.1136/jnnp-2011-301719
Calabrese EJ (2008) Converging concepts: adaptive response, preconditioning, and the Yerkes–Dodson Law are manifestations of hormesis. Ageing research reviews 7(1):8–20. https://doi.org/10.1016/j.arr.2007.07.001
Calabrese EJ, Mattson MP (2017) How does hormesis impact biology, toxicology, and medicine? NPJ Aging Mech Dis 3:13. https://doi.org/10.1038/s41514-017-0013-z
Cannon JR, Keep RF, Hua Y, Richardson RJ, Schallert T, Xi G (2005) Thrombin preconditioning provides protection in a 6-hydroxydopamine Parkinson’s disease model. Neurosci Lett 373(3):189–194
Cannon JR, Hua Y, Richardson RJ, Xi G, Keep RF, Schallert T (2007) The effect of thrombin on a 6-hydroxydopamine model of Parkinson’s disease depends on timing. Behav Brain Res 183(2):161–168. https://doi.org/10.1016/j.bbr.2007.06.004
Capper-Loup C, Frey CM, Rebell D, Kaelin-Lang A (2013) Adaptive gene expression changes on the healthy side of parkinsonian rats. Neuroscience 233:157–165. https://doi.org/10.1016/j.neuroscience.2012.12.027
Carvey PM, Punati A, Newman MB (2006) Progressive dopamine neuron loss in Parkinson’s disease: the multiple hit hypothesis. Cell Transplant 15(3):239–250
Chan CS, Gertler TS, Surmeier DJ (2010) A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov Disord 25(Suppl 1):S63–S70. https://doi.org/10.1002/mds.22801
Chen J, Simon R (1997) Ischemic tolerance in the brain. Neurology 48(2):306–311
Cohen AD, Zigmond MJ, Smith AD (2011) Effects of intrastriatal GDNF on the response of dopamine neurons to 6-hydroxydopamine: time course of protection and neurorestoration. Brain Res Brain Res Protoc 1370:80–88. https://doi.org/10.1016/j.brainres.2010.11.006
Cunha RA (2016) How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem. https://doi.org/10.1111/jnc.13724
de Lima Portella R, Lynn Bickta J, Shiva S (2015) Nitrite confers preconditioning and cytoprotection after ischemia/reperfusion injury through the modulation of mitochondrial function. Antioxid Redox Signal 23(4):307–327. https://doi.org/10.1089/ars.2015.6260
Dethy S, Van Blercom N, Damhaut P, Wikler D, Hildebrand J, Goldman S (1998) Asymmetry of basal ganglia glucose metabolism and dopa responsiveness in parkinsonism. Mov Disord 13(2):275–280. https://doi.org/10.1002/mds.870130213
Dirnagl U, Simon RP, Hallenbeck JM (2003) Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 26(5):248–254
Dirnagl U, Becker K, Meisel A (2009) Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 8(4):398–412. https://doi.org/10.1016/S1474-4422(09)70054-7
Djaldetti R, Ziv I, Melamed E (2006) The mystery of motor asymmetry in Parkinson’s disease. Lancet Neurol 5(9):796–802. https://doi.org/10.1016/S1474-4422(06)70549-X
Douglas R, Kellaway L, Mintz M, van Wageningen G (1987) The crossed nigrostriatal projection decussates in the ventral tegmental decussation. Brain Res 418(1):111–121
Fass B, Butcher LL (1981) Evidence for a crossed nigrostriatal pathway in rats. Neurosci Lett 22(2):109–113
Fornaguera J, Schwarting RK, Boix F, Huston JP (1993) Behavioral indices of moderate nigro-striatal 6-hydroxydopamine lesion: a preclinical Parkinson’s model. Synapse 13(2):179–185. https://doi.org/10.1002/syn.890130209
Fornaguera J, Carey RJ, Huston JP, Schwarting RK (1994) Behavioral asymmetries and recovery in rats with different degrees of unilateral striatal dopamine depletion. Brain Res 664(1–2):178–188
Fouillet A, Levet C, Virgone A, Robin M, Dourlen P, Rieusset J, Belaidi E, Ovize M, Touret M, Nataf S, Mollereau B (2012) ER stress inhibits neuronal death by promoting autophagy. Autophagy 8(6):915–926. https://doi.org/10.4161/auto.19716
Franklin KBJ, Paxinos G (2013) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates, 4th edn. Academic Press, Amsterdam
Geurts AC, Boonstra TA, Voermans NC, Diender MG, Weerdesteyn V, Bloem BR (2011) Assessment of postural asymmetry in mild to moderate Parkinson’s disease. Gait Posture 33(1):143–145. https://doi.org/10.1016/j.gaitpost.2010.09.018
Gidday JM (2006) Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci 7(6):437–448. https://doi.org/10.1038/nrn1927
Gleixner AM, Posimo JM, Pant DB, Henderson MP, Leak RK (2015) Astrocytes surviving severe stress can still protect neighboring neurons from proteotoxic injury. Mol Neurobiol. https://doi.org/10.1007/s12035-015-9427-4
Golpich M, Rahmani B, Mohamed Ibrahim N, Dargahi L, Mohamed Z, Raymond AA, Ahmadiani A (2015) Preconditioning as a potential strategy for the prevention of Parkinson’s disease. Mol Neurobiol 51(1):313–330. https://doi.org/10.1007/s12035-014-8689-6
Gu M, Owen AD, Toffa SE, Cooper JM, Dexter DT, Jenner P, Marsden CD, Schapira AH (1998) Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J Neurol Sci 158(1):24–29
Hamidi GA, Faraji A, Zarmehri HA, Haghdoost-Yazdi H (2012) Prolonged hyperoxia preconditioning attenuates behavioral symptoms of 6-hydroxydopamine-induced Parkinsonism. Neurol Res 34(7):636–642. https://doi.org/10.1179/1743132812Y.0000000056
Hara H, Kamiya T, Adachi T (2011) Endoplasmic reticulum stress inducers provide protection against 6-hydroxydopamine-induced cytotoxicity. Neurochem Int 58(1):35–43. https://doi.org/10.1016/j.neuint.2010.10.006
Harding DI, Greensmith L, Mason M, Anderson PN, Vrbova G (1999) Overexpression of GAP-43 induces prolonged sprouting and causes death of adult motoneurons. Eur J Neurosci 11(7):2237–2242
Hastings TG (2009) The role of dopamine oxidation in mitochondrial dysfunction: implications for Parkinson’s disease. J Bioenerg Biomembr 41(6):469–472. https://doi.org/10.1007/s10863-009-9257-z
Hawkes CH, Del Tredici K, Braak H (2007) Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33(6):599–614. https://doi.org/10.1111/j.1365-2990.2007.00874.x
Heinemann SD, Posimo JM, Mason DM, Hutchison DF, Leak RK (2016) Synergistic stress exacerbation in hippocampal neurons: evidence favoring the dual-hit hypothesis of neurodegeneration. Hippocampus. https://doi.org/10.1002/hipo.22580
Hobson DE (2012) Asymmetry in parkinsonism, spreading pathogens and the nose. Parkinsonism Relat Disord 18(1):1–9. https://doi.org/10.1016/j.parkreldis.2011.06.011
Hornykiewicz O, Kish SJ (1987) Biochemical pathophysiology of Parkinson’s disease. Adv Neurol 45:19–34
Hudson JL, van Horne CG, Stromberg I, Brock S, Clayton J, Masserano J, Hoffer BJ, Gerhardt GA (1993) Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res 626(1–2):167–174
Iancu R, Mohapel P, Brundin P, Paul G (2005) Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson’s disease in mice. Behav Brain Res 162(1):1–10. https://doi.org/10.1016/j.bbr.2005.02.023
Iwata SI, Nomoto M, Fukuda T (2001) Regulation of GAP-43 protein and mRNA in nigrostriatal dopaminergic neurons after the partial destruction of dopaminergic terminals with intrastriatal 6-hydroxydopamine. Synapse 39(1):16–22. 10.1002/1098-2396(20010101)39:1%3c16:AID-SYN3%3e3.0.CO;2-#
Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A (1999) A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods 93(2):149–162
Kirino T, Tsujita Y, Tamura A (1991) Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab 11(2):299–307. https://doi.org/10.1038/jcbfm.1991.62
Leak RK (2014) Adaptation and sensitization to proteotoxic stress. Dose Response 12(1):24–56. https://doi.org/10.2203/dose-response.13-016.Leak
Leak RK, Liou AK, Zigmond MJ (2006) Effect of sublethal 6-hydroxydopamine on the response to subsequent oxidative stress in dopaminergic cells: evidence for preconditioning. J Neurochem 99(4):1151–1163
Leak RK, Zigmond MJ, Liou AK (2008) Adaptation to chronic MG132 reduces oxidative toxicity by a CuZnSOD-dependent mechanism. J Neurochem 106(2):860–874
Lee M, Carroll TJ (2007) Cross education: possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med 37(1):1–14
Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ (2008) Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J Neurosci Res 86(1):108–117
Lindgren N, Leak RK, Carlson KM, Smith AD, Zigmond MJ (2008) Activation of the extracellular signal-regulated kinases 1 and 2 by glial cell line-derived neurotrophic factor and its relation to neuroprotection in a mouse model of Parkinson’s disease. J Neurosci Res 86(9):2039–2049
Ling Z, Zhu Y, Tong C, Snyder JA, Lipton JW, Carvey PM (2006) Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol 199(2):499–512. https://doi.org/10.1016/j.expneurol.2006.01.010
Lusardi TA, Farr CD, Faulkner CL, Pignataro G, Yang T, Lan J, Simon RP, Saugstad JA (2010) Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. J Cereb Blood Flow Metab 30(4):744–756. https://doi.org/10.1038/jcbfm.2009.253
Marinus J, van Hilten JJ (2015) The significance of motor (a)symmetry in Parkinson’s disease. Mov Disord 30(3):379–385. https://doi.org/10.1002/mds.26107
Mason DM, Nouraei N, Pant DB, Miner KM, Hutchison DF, Luk KC, Stolz JF, Leak RK (2016) Transmission of alpha-synucleinopathy from olfactory structures deep into the temporal lobe. Mol Neurodegener 11(1):49. https://doi.org/10.1186/s13024-016-0113-4
Mattson MP, Duan W, Wan R, Guo Z (2004) Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx 1(1):111–116. https://doi.org/10.1602/neurorx.1.1.111
Matus S, Castillo K, Hetz C (2012) Hormesis: protecting neurons against cellular stress in Parkinson disease. Autophagy 8(6):997–1001. https://doi.org/10.4161/auto.20748
Miklyaeva EI, Castaneda E, Whishaw IQ (1994) Skilled reaching deficits in unilateral dopamine-depleted rats: impairments in movement and posture and compensatory adjustments. J Neurosci 14(11 Pt 2):7148–7158
Miklyaeva EI, Woodward NC, Nikiforov EG, Tompkins GJ, Klassen F, Ioffe ME, Whishaw IQ (1997) The ground reaction forces of postural adjustments during skilled reaching in unilateral dopamine-depleted hemiparkinson rats. Behav Brain Res 88(2):143–152
Miklyaeva EI, Whishaw IQ, Kolb B (2007) A golgi analysis of cortical pyramidal cells in the unilateral parkinson rat: absence of change in the affected hemisphere vs hypertrophy in the intact hemisphere. Restor Neurol Neurosci 25(2):91–99
Mollereau B, Rzechorzek NM, Roussel BD, Sedru M, Van den Brink DM, Bailly-Maitre B, Palladino F, Medinas DB, Domingos PM, Hunot S, Chandran S, Birman S, Baron T, Vivien D, Duarte CB, Ryoo HD, Steller H, Urano F, Chevet E, Kroemer G, Ciechanover A, Calabrese EJ, Kaufman RJ, Hetz C (2016) Adaptive preconditioning in neurological diseases—therapeutic insights from proteostatic perturbations. Brain Res 1648(Pt B):603–616. https://doi.org/10.1016/j.brainres.2016.02.033
Morgan S, Huston JP, Pritzel M (1983) Effects of reducing sensory-motor feedback on the appearance of crossed nigro-thalamic projections and recovery from turning induced by unilateral substantia nigra lesions. Brain Res Bull 11(6):721–727
Morgan S, Nomikos G, Huston JP (1991) Changes in the nigrostriatal projection associated with recovery from lesion-induced behavioral asymmetry. Behav Brain Res 46(2):157–165
Nakajima K, Hida H, Shimano Y, Fujimoto I, Hashitani T, Kumazaki M, Sakurai T, Nishino H (2001) GDNF is a major component of trophic activity in DA-depleted striatum for survival and neurite extension of DAergic neurons. Brain Res 916(1–2):76–84
Nikolaus S, Larisch R, Beu M, Forutan F, Vosberg H, Muller-Gartner HW (2003) Bilateral increase in striatal dopamine D2 receptor density in the 6-hydroxydopamine-lesioned rat: a serial in vivo investigation with small animal PET. Eur J Nucl Med Mol Imaging 30(3):390–395. https://doi.org/10.1007/s00259-002-1056-2
Nouraei N, Zarger L, Weilnau JN, Han J, Mason DM, Leak RK (2016) Investigation of the therapeutic potential of N-acetyl cysteine and the tools used to define nigrostriatal degeneration in vivo. Toxicol Appl Pharmacol 296:19–30. https://doi.org/10.1016/j.taap.2016.02.010
Obeso JA, Rodriguez-Oroz MC, Lanciego JL, Rodriguez Diaz M (2004) How does Parkinson’s disease begin? The role of compensatory mechanisms. Trends Neurosci 27(3):125–127. https://doi.org/10.1016/j.tins.2003.12.006 (author reply 127–128)
Penit-Soria J, Durand C, Herve D, Besson MJ (1997) Morphological and biochemical adaptations to unilateral dopamine denervation of the neostriatum in newborn rats. Neuroscience 77(3):753–766
Perea G, Sur M, Araque A (2014) Neuron-glia networks: integral gear of brain function. Front Cell Neurosci 8:378. https://doi.org/10.3389/fncel.2014.00378
Perovic M, Mladenovic A, Rakic L, Ruzdijic S, Kanazir S (2005) Increase of GAP-43 in the rat cerebellum following unilateral striatal 6-OHDA lesion. Synapse 56(3):170–174. https://doi.org/10.1002/syn.20142
Pritzel M, Huston JP (1981) Neural and behavioral plasticity: crossed nigro-thalamic projections following unilateral substantia nigra lesions. Behav Brain Res 3(3):393–399
Pritzel M, Huston JP, Sarter M (1983) Behavioral and neuronal reorganization after unilateral substantia nigra lesions: evidence for increased interhemispheric nigrostriatal projections. Neuroscience 9(4):879–888
Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M (2014) Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res 114(10):1601–1610. https://doi.org/10.1161/CIRCRESAHA.114.303822
Riederer P, Sian-Hulsmann J (2012) The significance of neuronal lateralisation in Parkinson’s disease. J Neural Transm (Vienna) 119(8):953–962. https://doi.org/10.1007/s00702-012-0775-1
Robertson GS, Robertson HA (1989) Evidence that l-dopa-induced rotational behavior is dependent on both striatal and nigral mechanisms. J Neurosci 9(9):3326–3331
Robinson TE, Castaneda E, Whishaw IQ (1990) Compensatory changes in striatal dopamine neurons following recovery from injury induced by 6-OHDA or methamphetamine: a review of evidence from microdialysis studies. Can J Psychol 44(2):253–275
Roedter A, Winkler C, Samii M, Walter GF, Brandis A, Nikkhah G (2001) Comparison of unilateral and bilateral intrastriatal 6-hydroxydopamine-induced axon terminal lesions: evidence for interhemispheric functional coupling of the two nigrostriatal pathways. J Comp Neurol 432(2):217–229
Roemmich RT, Nocera JR, Stegemoller EL, Hassan A, Okun MS, Hass CJ (2014) Locomotor adaptation and locomotor adaptive learning in Parkinson’s disease and normal aging. Clin Neurophysiol 125(2):313–319. https://doi.org/10.1016/j.clinph.2013.07.003
Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M, Tremblay R, Kiuchi K, Sikorska M (2009) Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity. Neurobiol Dis 33(3):405–414. https://doi.org/10.1016/j.nbd.2008.11.016
Sauer H, Oertel WH (1994) Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience 59(2):401–415
Saura J, Pares M, Bove J, Pezzi S, Alberch J, Marin C, Tolosa E, Marti MJ (2003) Intranigral infusion of interleukin-1beta activates astrocytes and protects from subsequent 6-hydroxydopamine neurotoxicity. J Neurochem 85(3):651–661
Schallert T, Jones TA (1993) “Exuberant” neuronal growth after brain damage in adult rats: the essential role of behavioral experience. J Neural Transplant Plast 4(3):193–198. https://doi.org/10.1155/NP.1993.193
Schallert T, Upchurch M, Wilcox RE, Vaughn DM (1983) Posture-independent sensorimotor analysis of inter-hemispheric receptor asymmetries in neostriatum. Pharmacol Biochem Behav 18(5):753–759
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 39(5):777–787
Schumacher L, Urbach A, Lutzenburg M, Bidmon HJ, Witte OW (2014) Bihemispheric ischemic tolerance induced by a unilateral focal cortical lesion. Brain Res 1570:54–60. https://doi.org/10.1016/j.brainres.2014.05.008
Schwarting RK, Huston JP (1996a) The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol 50(2–3):275–331
Schwarting RK, Huston JP (1996b) Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Prog Neurobiol 49(3):215–266
Schwarting RK, Bonatz AE, Carey RJ, Huston JP (1991) Relationships between indices of behavioral asymmetries and neurochemical changes following mesencephalic 6-hydroxydopamine injections. Brain Res 554(1–2):46–55
Selye H (1975) Stress without distress. Signet, Philadelphia
Smeyne M, Sladen P, Jiao Y, Dragatsis I, Smeyne RJ (2015) HIF1alpha is necessary for exercise-induced neuroprotection while HIF2alpha is needed for dopaminergic neuron survival in the substantia nigra pars compacta. Neuroscience 295:23–38. https://doi.org/10.1016/j.neuroscience.2015.03.015
Smith AD, Amalric M, Koob GF, Zigmond MJ (2002) Effect of bilateral 6-hydroxydopamine lesions of the medial forebrain bundle on reaction time. Neuropsychopharmacology 26(6):756–764. https://doi.org/10.1016/S0893-133X(01)00420-1
Smith AD, Antion M, Zigmond MJ, Austin MC (2003) Effect of 6-hydroxydopamine on striatal GDNF and nigral GFRalpha1 and RET mRNAs in the adult rat. Brain Res Mol Brain Res 117(2):129–138
Steiner H, Morgan S, Huston JP (1985) Effect of forebrain commissurotomy on recovery from unilateral 6-OHDA lesions of the substantia nigra and circling induced by apomorphine. Behav Brain Res 17(3):245–249
Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, Gao Y (2014) Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Prog Neurobiol 114:58–83. https://doi.org/10.1016/j.pneurobio.2013.11.005
Stochl J, Hagtvet KA, Brozova H, Klempir J, Roth J, Ruzicka E (2009) Handedness does not predict side of onset of motor symptoms in Parkinson’s disease. Mov Disord 24(12):1836–1839. https://doi.org/10.1002/mds.22653
Stokes AH, Hastings TG, Vrana KE (1999) Cytotoxic and genotoxic potential of dopamine. J Neurosci Res 55(6):659–665
Stott SR, Barker RA (2014) Time course of dopamine neuron loss and glial response in the 6-OHDA striatal mouse model of Parkinson’s disease. Eur J Neurosci 39(6):1042–1056. https://doi.org/10.1111/ejn.12459
Thiele SL, Warre R, Nash JE (2012) Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson’s disease. J Vis Exp 60:3234. https://doi.org/10.3791/3234
Titler AM, Posimo JM, Leak RK (2013) Astrocyte plasticity revealed by adaptations to severe proteotoxic stress. Cell Tissue Res 352(3):427–443. https://doi.org/10.1007/s00441-013-1571-4
Ungerstedt U (1968) 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 5(1):107–110
Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res 24(3):485–493
Unnithan AS, Choi HJ, Titler AM, Posimo JM, Leak RK (2012) Rescue from a two hit, high-throughput model of neurodegeneration with N-acetyl cysteine. Neurochem Int 61(3):356–368. https://doi.org/10.1016/j.neuint.2012.06.001
Unnithan AS, Jiang Y, Rumble JL, Pulugulla SH, Posimo JM, Gleixner AM, Leak RK (2014) N-acetyl cysteine prevents synergistic, severe toxicity from two hits of oxidative stress. Neurosci Lett 560:71–76. https://doi.org/10.1016/j.neulet.2013.12.023
Valdes P, Mercado G, Vidal RL, Molina C, Parsons G, Court FA, Martinez A, Galleguillos D, Armentano D, Schneider BL, Hetz C (2014) Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc Natl Acad Sci USA 111(18):6804–6809. https://doi.org/10.1073/pnas.1321845111
van der Hoorn A, Bartels AL, Leenders KL, de Jong BM (2011) Handedness and dominant side of symptoms in Parkinson’s disease. Parkinsonism Relat Disord 17(1):58–60. https://doi.org/10.1016/j.parkreldis.2010.10.002
van der Hoorn A, Burger H, Leenders KL, de Jong BM (2012) Handedness correlates with the dominant Parkinson side: a systematic review and meta-analysis. Mov Disord 27(2):206–210. https://doi.org/10.1002/mds.24007
Von Voigtlander PF, Moore KE (1973) Turning behavior of mice with unilateral 6-hydroxydopamine lesions in the striatum: effects of apomorphine, l-DOPA, amanthadine, amphetamine and other psychomotor stimulants. Neuropharmacology 12(5):451–462
Vos PE, Steinbusch HW, Ronken E, van Ree JM (1999) Short and long term plasticity after lesioning of the cell body or terminal field area of the dopaminergic mesocorticolimbic system in the rat. Brain Res 831(1–2):237–247
Walsh S, Finn DP, Dowd E (2011) Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson’s disease in the rat. Neuroscience 175:251–261. https://doi.org/10.1016/j.neuroscience.2010.12.005
Wang Z, Liu J, Chen S, Wang Y, Cao L, Zhang Y, Kang W, Li H, Gui Y, Chen S, Ding J (2011) DJ-1 modulates the expression of Cu/Zn-superoxide dismutase-1 through the Erk1/2-Elk1 pathway in neuroprotection. Ann Neurol 70(4):591–599. https://doi.org/10.1002/ana.22514
Wang J, Yang QX, Sun X, Vesek J, Mosher Z, Vasavada M, Chu J, Kanekar S, Shivkumar V, Venkiteswaran K, Subramanian T (2015) MRI evaluation of asymmetry of nigrostriatal damage in the early stage of early-onset Parkinson’s disease. Parkinsonism Relat Disord 21(6):590–596. https://doi.org/10.1016/j.parkreldis.2015.03.012
Watson RE Jr, Wiegand SJ, Clough RW, Hoffman GE (1986) Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7(1):155–159
Woodlee MT, Kane JR, Chang J, Cormack LK, Schallert T (2008) Enhanced function in the good forelimb of hemi-parkinson rats: compensatory adaptation for contralateral postural instability? Exp Neurol 211(2):511–517. https://doi.org/10.1016/j.expneurol.2008.02.024
Woods SC, Ramsay DS (2007) Homeostasis: beyond Curt Richter. Appetite 49(2):388–398. https://doi.org/10.1016/j.appet.2006.09.015
Yuan H, Sarre S, Ebinger G, Michotte Y (2005) Histological, behavioural and neurochemical evaluation of medial forebrain bundle and striatal 6-OHDA lesions as rat models of Parkinson’s disease. J Neurosci Methods 144(1):35–45. https://doi.org/10.1016/j.jneumeth.2004.10.004
Zhang J, Yang ZJ, Klaus JA, Koehler RC, Huang J (2008) Delayed tolerance with repetitive transient focal ischemic preconditioning in the mouse. Stroke 39(3):967–974. https://doi.org/10.1161/STROKEAHA.107.497412
Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM (1990) Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci 13(7):290–296
Zurkovsky L, Bychkov E, Tsakem EL, Siedlecki C, Blakely RD, Gurevich EV (2013) Cognitive effects of dopamine depletion in the context of diminished acetylcholine signaling capacity in mice. Dis Model Mech 6(1):171–183. https://doi.org/10.1242/dmm.010363
Acknowledgements
Designed the experiments and wrote the paper: RKL. Performed the experiments and generated the figures: JW. Analyzed the data: JW, MC, KM, TB, DH, DP, and NN. We are grateful to Deborah Willson and Jackie Farrer for excellent administrative support and the Denise Butler-Buccilli and Christine Close for outstanding animal care. Funded by awards to RKL from the Hillman Family Foundation (GRANT109033) and the National Institutes of Health (R15NS093539).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
429_2017_1552_MOESM1_ESM.tif
Supplemental Fig. 1: Reproducibility of motor and histological assessments. Measurements shown in graphs from the main text were repeated by a blinded observer. (A-B) The second blinded assessment of landing behavior was consistent with the first blinded assessment shown in Fig. 1E-F of the main text. The second assessments of (C) striatal TH and (D) striatal DAT were consistent with the first assessments shown in Figs. 3C and 3F, respectively. (E) The second assessment of nigral cell numbers was consistent with the first assessments shown in Fig. 4E. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001 left versus right hemisphere or turns; a p ≤ 0.05, aa p ≤ 0.01 versus group a; b p ≤ 0.05, bb p ≤ 0.01, bbb p ≤ 0.001 versus group b; two-way ANOVA followed by Bonferroni post hoc correction (TIFF 1146 kb)
429_2017_1552_MOESM2_ESM.tif
Supplemental Fig. 2: Raw measurements of two independent dopaminergic markers in the striatum. Tyrosine hydroxylase (TH) and dopamine transporter (DAT) measurements shown in the main text in Figs. 3A and 3E were based on the raw immunofluorescent values shown here in panels A and B, respectively. Note that the effect sizes and p values are identical for the raw and percentage data. *** p ≤ 0.001 left versus right hemisphere; a p ≤ 0.05, aa p ≤ 0.01, aaa p ≤ 0.001 versus group a; bb p ≤ 0.01, bbb p ≤ 0.001 versus group b; two-way ANOVA followed by Bonferroni post hoc correction (TIFF 695 kb)
429_2017_1552_MOESM3_ESM.tiff
Supplemental Fig. 3: Zoomable version of Fig. 4. See main text for legend. Note that the images of the ventral midbrain are very large in size because each montage was stitched together from multiple high-resolution photos captured with a 10 × objective. Therefore, the cellular features in the photomicrograph take time to populate on the computer screen, sometimes up to several minutes. Note that there may be computer-introduced imperfections at the boundaries of the stitches. All tissue was processed in parallel with the same solutions. Images from all four groups were captured at the same exposure and intensity scaling. The intensity scaling is relatively high so that even weakly labeled TH+ cells can be visualized (TIFF 60145 kb)
Rights and permissions
About this article
Cite this article
Weilnau, J.N., Carcella, M.A., Miner, K.M. et al. Evidence for cross-hemispheric preconditioning in experimental Parkinson’s disease. Brain Struct Funct 223, 1255–1273 (2018). https://doi.org/10.1007/s00429-017-1552-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1552-6