Abstract
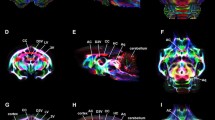
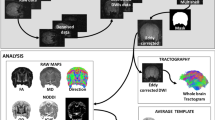
Tree shrews are small mammals now commonly classified in the order of Scandentia, but have relatively closer affinity to primates than rodents. The species has a high brain-to-body mass ratio and relatively well-differentiated neocortex, and thus has been frequently used in neuroscience research, especially for studies on vision and neurological/psychiatric diseases. The available atlases on tree shrew brain provided only limited information on white matter (WM) anatomy. In this study, diffusion tensor imaging (DTI) was used to study the WM anatomy of tree shrew, with the goal to establish an image-based WM atlas. DTI and T2-weighted anatomical images were acquired in vivo and from fixed brain samples. Deterministic tractography was used for three-dimensional reconstruction and rendering of major WM tracts. Myelin and neurofilaments staining were used to study the microstructural properties of certain WM tracts. Taking into account prior knowledge on tree shrew neuroanatomy, tractography results, and comparisons to the homologous structures in rodents and primates, an image-based WM atlas of tree shrew brain was constructed, which is available to research community upon request.








Similar content being viewed by others
References
Aboitiz F, Garcia VR (1997) The evolutionary origin of the language areas in the human brain. A neuroanatomical perspective. Brain Res Rev 25:381–396
Adluru N, Zhang H, Fox AS et al (2012) A diffusion tensor brain template for rhesus macaques. Neuroimage 59:306–318
Aggarwal M, Nauen DW, Troncoso JC et al (2015) Probing region-specific microstructure of human cortical areas using high angular and spatial resolution diffusion MRI. Neuroimage 105:198–207
Atlan G, Terem A, Peretz-Rivlin N et al (2016) Mapping synaptic cortico-claustral connectivity in the mouse. J Comp Neurol. doi:10.1002/cne.23997
Avants BB, Tustison NJ, Song G et al (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 54:2033–2044
Bedwell SA, Billett EE, Crofts JJ et al (2015) The topology of connections between rat prefrontal and temporal cortices. Front Syst Neurosci 9:80
Behrens TE, Berg HJ, Jbabdi S et al (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34:144–155
Benveniste H, Einstein G, Kim KR et al (1999) Detection of neuritic plaques in Alzheimer’s disease by magnetic resonance microscopy. Proc Natl Acad Sci USA 96:14079–14084
Berke JJ (1960) The claustrum, the external capsule and the extreme capsule of Macaca mulatta. J Comp Neurol 115:297–331
Bora E, Yucel M, Fornito A et al (2012) White matter microstructure in opiate addiction. Addict Biol 17:141–148
Bosking WH, Zhang Y, Schofield B et al (1997) Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci 17:2112–2127
Calabrese E, Badea A, Coe CL et al (2015) A diffusion tensor MRI atlas of the postmortem rhesus macaque brain. Neuroimage 117:408–416
Campbell CB, Jane JA, Yashon D (1967) The retinal projections of the tree shrew and hedgehog. Brain Res 5:406–418
Cao J, Yang EB, Su JJ et al (2003) The tree shrews: adjuncts and alternatives to primates as models for biomedical research. J Med Primatol 32:123–130
Carey RG, Neal TL (1986) Reciprocal connections between the claustrum and visual thalamus in the tree shrew (tupaia-glis). Brain Res 386:155–168
Carey RG, Fitzpatrick D, Diamond IT (1979) Layer I of striate cortex of tupaia glis and galago senegalensis: projections from thalamus and claustrum revealed by retrograde transport of horseradish peroxidase. J Comp Neurol 186:393–437
Carey RG, Bear MF, Diamond IT (1980) Laminar organization of the reciprocal projections between the claustrum and striate cortex in the tree shrew, tupaia-glis. Brain Res 184:193–198
Casseday HJ, Diamond IT, Harting JK (1976) Auditory pathways to the cortex in tupaia glis. J Comp Neurol 166:303–340
Catani M, Howard RJ, Pajevic S et al (2002) Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17:77–94
Chan E, Kovacevic N, Ho SK et al (2007) Development of a high resolution three-dimensional surgical atlas of the murine head for strains 129S1/SvImJ and C57Bl/6J using magnetic resonance imaging and micro-computed tomography. Neuroscience 144:604–615
Chomsung RD, Petry HM, Bickford ME (2008) Ultrastructural examination of diffuse and specific tectopulvinar projections in the tree shrew. J Comp Neurol 510:24–46
Chomsung RD, Wei H, Day-Brown JD et al (2010) Synaptic organization of connections between the temporal cortex and pulvinar nucleus of the tree shrew. Cereb Cortex 20:997–1011
Chuang N, Mori S, Yamamoto A et al (2011) An MRI-based atlas and database of the developing mouse brain. Neuroimage 54:80–89
Conturo TE, Lori NF, Cull TS et al (1999) Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA 96:10422–10427
Dalby RB, Frandsen J, Chakravarty MM et al (2010) Depression severity is correlated to the integrity of white matter fiber tracts in late-onset major depression. Psychiatry Res 184:38–48
Day-Brown JD, Wei H, Chomsung RD et al (2010) Pulvinar projections to the striatum and amygdala in the tree shrew. Front Neuroanat 4:143
Delatour B, Witter MP (2002) Projections from the parahippocampal region to the prefrontal cortex in the rat: evidence of multiple pathways. Eur J Neurosci 15:1400–1407
Fan Y, Huang ZY, Cao CC et al (2013) Genome of the Chinese tree shrew. Nat Commun 4:1426
Figini M, Zucca I, Aquino D et al (2015) In vivo DTI tractography of the rat brain: an atlas of the main tracts in Paxinos space with histological comparison. Magn Reson Imaging 33:296–303
Fitzpatrick D (1996) The functional organization of local circuits in visual cortex: insights from the study of tree shrew striate cortex. Cereb Cortex 6:329–341
Fitzpatrick D, Carey RG, Diamond IT (1980) The projection of the superior colliculus upon the lateral geniculate-body in tupaia-glis and galago-senegalensis. Brain Res 194:494–499
Flugge G, Ahrens O, Fuchs E (1994) Monoamine receptors in the amygdaloid complex of the tree shrew (tupaia belangeri). J Comp Neurol 343:597–608
Fox AS, Oler JA, do Tromp PM et al (2015) Extending the amygdala in theories of threat processing. Trends Neurosci 38:319–329
Foxley S, Jbabdi S, Clare S et al (2014) Improving diffusion-weighted imaging of post-mortem human brains: SSFP at 7 T. Neuroimage 102(Pt 2):579–589
Fuchs E (2005) Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectr 10:182–190
Fuchs E, Flugge G (2002) Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacol Biochem Behav 73:247–258
Glickstein M (1967) Laminar structure of the dorsal lateral geniculate nucleus in the tree shrew (tupaia glis). J Comp Neurol 131:93–102
Hall WC, Lee P (1993) Interlaminar connections of the superior colliculus in the tree shrew. I. The superficial gray layer. J Comp Neurol 332:213–223
Hall WC, Lee P (1997) Interlaminar connections of the superior colliculus in the tree shrew. III: The optic layer. Vis Neurosci 14:647–661
Harting JK, Hall WC, Diamond IT et al (1973) Anterograde degeneration study of the superior colliculus in tupaia glis: evidence for a subdivision between superficial and deep layers. J Comp Neurol 148:361–386
Harting JK, Huerta MF, Hashikawa T et al (1991) Projection of the mammalian superior colliculus upon the dorsal lateral geniculate nucleus: organization of tectogeniculate pathways in nineteen species. J Comp Neurol 304:275–306
Hoover WB, Vertes RP (2011) Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol 519:3766–3801
Jain N, Preuss TM, Kaas JH (1994) Subdivisions of the visual system labeled with the Cat-301 antibody in tree shrews. Vis Neurosci 11:731–741
Jbabdi S, Johansen-Berg H (2011) Tractography: where do we go from here? Brain Connect 1:169–183
Kaas JH (2011) The evolution of auditory cortex: the core areas. In: Jeffery A, Winer CES (eds) The auditory cortex. Springer, US, pp 407–427
Keuker JI, de Biurrun G, Luiten PG et al (2004) Preservation of hippocampal neuron numbers and hippocampal subfield volumes in behaviorally characterized aged tree shrews. J Comp Neurol 468:509–517
Kowianski P, Dziewiatkowski J, Kowianska J et al (1999) Comparative anatomy of the claustrum in selected species: a morphometric analysis. Brain Behav Evol 53:44–54
Lee P, Hall WC (1995) Interlaminar connections of the superior colliculus in the tree shrew. II: projections from the superficial gray to the optic layer. Vis Neurosci 12:573–588
Lende RA (1970) Cortical localization in the tree shrew (tupaia). Brain Res 18:61–75
Li Q, Ni X (2016) An early Oligocene fossil demonstrates treeshrews are slowly evolving “living fossils”. Sci Rep 6:18627
Liu FG, Miyamoto MM, Freire NP et al (2001) Molecular and morphological supertrees for eutherian (placental) mammals. Science 291:1786–1789
Luppino G, Matelli M, Carey RG et al (1988) New view of the organization of the pulvinar nucleus in tupaia as revealed by tectopulvinar and pulvinar-cortical projections. J Comp Neurol 273:67–86
Lyon DC, Jain N, Kaas JH (2003a) The visual pulvinar in tree shrews I. Multiple subdivisions revealed through acetylcholinesterase and Cat-301 chemoarchitecture. J Comp Neurol 467:593–606
Lyon DC, Jain N, Kaas JH (2003b) The visual pulvinar in tree shrews II. Projections of four nuclei to areas of visual cortex. J Comp Neurol 467:607–627
Ma KL, Gao JH, Huang ZQ et al (2013) Motor function in MPTP-treated tree shrews (tupaia belangeri chinensis). Neurochem Res 38:1935–1940
Makris N, Pandya DN (2009) The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct 213:343–358
Marrocco RT, De Valois RL, Boles JI (1970) A stereotaxic atlas of the brain of the tree shrew (tupaia glis). J Hirnforsch 12:307–312
Mars RB, Foxley S, Verhagen L et al (2015) The extreme capsule fiber complex in humans and macaque monkeys: a comparative diffusion MRI tractography study. Brain Struct Funct. doi:10.1007/s00429-015-1146-0
Mathur BN (2014) The claustrum in review. Front Syst Neurosci 8:48
Matsuo K, Mizuno T, Yamada K et al (2008) Cerebral white matter damage in frontotemporal dementia assessed by diffusion tensor tractography. Neuroradiology 50:605–611
May PJ (2006) The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151:321–378
McCollum LA, Roberts RC (2014) Ultrastructural localization of tyrosine hydroxylase in tree shrew nucleus accumbens core and shell. Neuroscience 271:23–34
Mori S, van Zijl PC (2002) Fiber tracking: principles and strategies—a technical review. NMR Biomed 15:468–480
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539
Mori S, Oishi K, Jiang H et al (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40:570–582
Murphy WJ, Eizirik E, O’Brien SJ et al (2001) Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science 294:2348–2351
Ohl F, Kirschbaum C, Fuchs E (1999) Evaluation of hypothalamo-pituitary-adrenal activity in the tree shrew (tupaia belangeri) via salivary cortisol measurement. Lab Anim 33:269–274
Ohl F, Michaelis T, Vollmann-Honsdorf GK et al (2000) Effect of chronic psychosocial stress and long-term cortisol treatment on hippocampus-mediated memory and hippocampal volume: a pilot-study in tree shrews. Psychoneuroendocrinology 25:357–363
Ongur D, Price JL (2000) The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex 10:206–219
Pajevic S, Pierpaoli C (1999) Color schemes to represent the orientation of anisotropic tissues from diffusion tensor data: application to white matter fiber tract mapping in the human brain. Magn Reson Med 42:526–540
Palchaudhuri M, Flugge G (2005) 5-HT1A receptor expression in pyramidal neurons of cortical and limbic brain regions. Cell Tissue Res 321:159–172
Park S, Tyszka JM, Allman JM (2012) The claustrum and insula in microcebus murinus: a high resolution diffusion imaging study. Front Neuroanat 6:21
Pawlik M, Fuchs E, Walker LC et al (1999) Primate-like amyloid-β sequence but no cerebral amyloidosis in aged tree shrews. Neurobiol Aging 20:47–51
Paxinos G, Watson C (2006) The rat brain in stereotaxic coordinates, 6th edn. Academic Press, San Diego
Peng Y, Ye Z, Zou R et al (1991) Biology of Chinese tree shrews. Yunnan Science and Technology Press, Kunming
Petros TJ, Rebsam A, Mason CA (2008) Retinal axon growth at the optic chiasm: to cross or not to cross. Annu Rev Neurosci 31:295–315
Poletti CE, Creswell G (1977) Fornix system efferent projections in the squirrel monkey: an experimental degeneration study. J Comp Neurol 175:101–128
Pritzel M, Kretz R, Rager G (1988) Callosal projections between areas-17 in the adult tree shrew (tupaia-belangeri). Exp Brain Res 72:481–493
Remple MS, Reed JL, Stepniewska I et al (2006) Organization of frontoparietal cortex in the tree shrew (tupaia belangeri). I. Architecture, microelectrode maps, and corticospinal connections. J Comp Neurol 497:133–154
Remple MS, Reed JL, Stepniewska I et al (2007) The organization of frontoparietal cortex in the tree shrew (tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J Comp Neurol 501:121–149
Rice MW, Roberts RC, Melendez-Ferro M et al (2011) Neurochemical characterization of the tree shrew dorsal striatum. Front Neuroanat 5:53
Rilling JK, Glasser MF, Preuss TM et al (2008) The evolution of the arcuate fasciculus revealed with comparative DTI. Nat Neurosci 11:426–428
Rilling JK, Glasser MF, Jbabdi S et al (2011) Continuity, divergence, and the evolution of brain language pathways. Front Evol Neurosci 3:11
Sati P, Silva AC, van Gelderen P et al (2012) In vivo quantification of T(2) anisotropy in white matter fibers in marmoset monkeys. Neuroimage 59:979–985
Saur D, Kreher BW, Schnell S et al (2008) Ventral and dorsal pathways for language. Proc Natl Acad Sci USA 105:18035–18040
Schmahmann JD, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press, New York
Schmahmann JD, Pandya DN, Wang R et al (2007) Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653
Shen F, Duan Y, Jin S et al (2014) Varied behavioral responses induced by morphine in the tree shrew: a possible model for human opiate addiction. Front Behav Neurosci 8:333
Shenton ME, Hamoda HM, Schneiderman JS et al (2012) A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav 6:137–192
Shibata S, Komaki Y, Seki F et al (2015) Connectomics: comprehensive approaches for whole-brain mapping. Microscopy 64:57–67
Sillitoe RV, Malz CR, Rockland K et al (2004) Antigenic compartmentation of the primate and tree shrew cerebellum: a common topography of zebrin II in macaca mulatta and tupaia belangeri. J Anat 204:257–269
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Thiebaut de Schotten M, Dell’Acqua F, Valabregue R et al (2012) Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48:82–96
Thomas C, Ye FQ, Irfanoglu MO et al (2014) Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc Natl Acad Sci USA 111:16574–16579
Tigges J, Shantha TR (1969) A stereotaxic brain atlas of the tree shrew (tupaia glis). Williams & Wilkins, Baltimore
Tournier JD, Calamante F, Connelly A (2012) MRtrix: diffusion tractography in crossing fiber regions. Int J Imag Syst Tech 22:53–66
Wakana S, Jiang H, Nagae-Poetscher LM et al (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87
Wang S, Shan D, Dai J et al (2013) Anatomical MRI templates of tree shrew brain for volumetric analysis and voxel-based morphometry. J Neurosci Methods 220:9–17
Wong P, Kaas JH (2009) Architectonic subdivisions of neocortex in the tree shrew (tupaia belangeri). Anat Rec (Hoboken) 292:994–1027
Yamashita A, Fuchs E, Taira M et al (2010) Amyloid beta (Abeta) protein- and amyloid precursor protein (APP)-immunoreactive structures in the brains of aged tree shrews. Curr Aging Sci 3:230–238
Yamashita A, Fuchs E, Taira M et al (2012) Somatostatin-immunoreactive senile plaque-like structures in the frontal cortex and nucleus accumbens of aged tree shrews and Japanese macaques. J Med Primatol 41:147–157
Yang W, Liu J (1990) A stereotaxic atlas of the brain of tupaia belangeri and macaque monkey living in Guangxi. Guangxi Science and Technology Publishing House, Guangxi
Zambello E, Fuchs E, Abumaria N et al (2010) Chronic psychosocial stress alters NPY system: different effects in rat and tree shrew. Prog Neuropsychopharmacol Biol Psychiatry 34:122–130
Zhang H, Yushkevich PA, Rueckert D et al (2007) Unbiased white matter atlas construction using diffusion tensor images. Med Image Comput Comput Assist Interv 4792:211–218
Zilles K (1978) A quantitative approach to cytoarchitectonics. I. The areal pattern of the cortex of tupaia belangeri. Anat Embryol (Berl) 153:195–212
Zuo N, Fang J, Lv X et al (2012) White matter abnormalities in major depression: a tract-based spatial statistics and rumination study. PLoS One 7:e37561
Acknowledgments
The authors thank Dr. Yun-ling Gao for her assistance in histological staining. This work was supported by Grants from Chinese Ministry of Science and Technology (2011CB707800) and Natural Science Foundation of China (81171302 and 21221064).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, Jk., Wang, Sx., Shan, D. et al. A diffusion tensor imaging atlas of white matter in tree shrew. Brain Struct Funct 222, 1733–1751 (2017). https://doi.org/10.1007/s00429-016-1304-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1304-z