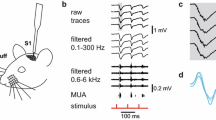
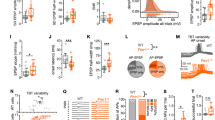
Abstract
Schizophrenic patients show altered sensory perception as well as changes in electrical and magnetic brain responses to sustained, frequency-modulated sensory stimulation. Both the amplitude and temporal precision of the neural responses differ in patients as compared to control subjects, and these changes are most pronounced for stimulation at gamma frequencies (20–40 Hz). In addition, patients display enhanced spontaneous gamma oscillations, which has been interpreted as ‘neural noise’ that may interfere with normal stimulus processing. To investigate electrophysiological markers of aberrant sensory processing in a model of schizophrenia, we recorded neuronal activity in primary somatosensory cortex of mice heterozygous for the schizophrenia susceptibility gene Neuregulin 1. Sensory responses to sustained 20–70 Hz whisker stimulation were analyzed with respect to firing rates, spike precision (phase locking) and gamma oscillations, and compared to baseline conditions. The mutants displayed elevated spontaneous firing rates, a reduced gain in sensory-evoked spiking and gamma activity, and reduced spike precision of 20–40 Hz responses. These findings present the first in vivo evidence of the linkage between a genetic marker and altered stimulus encoding, thus suggesting a novel electrophysiological endophenotype of schizophrenia.



Similar content being viewed by others
References
Agim ZS et al (2013) Discovery, validation and characterization of Erbb4 and Nrg1 haplotypes using data from three genome-wide association studies of schizophrenia. PLoS One 8:3
Andersson RH et al (2012) Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proc Natl Acad Sci USA 109:13118–13123
Arnfred SM (2012) Proprioceptive information processing in schizophrenia. Dan Med J 59
Arnfred SM, Hemmingsen RP, Parnas J (2006) Delayed early proprioceptive information processing in schizophrenia. Br J Psychiatry 189:558–559
Arnfred SM, Morup M, Thalbitzer J, Jansson L, Parnas J (2011) Attenuation of beta and gamma oscillations in schizophrenia spectrum patients following hand posture perturbation. Psychiatry Res 185:215–224
Bakker SC, Hoogendoorn MLC, Selten JP, Verduijn W, Pearson PL, Sinke RJ, Kahn RS (2004) Neuregulin 1: genetic support for schizophrenia subtypes. Mol Psychiatry 9:1061–1063
Bartos M, Vida I, Jonas P (2007) Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 8:45–56
Becker K, Eder M, Ranft A, von Meyer L, Zieglgansberger W, Kochs E, Dodt HU (2012) Low dose isoflurane exerts opposing effects on neuronal network excitability in neocortex and hippocampus PLoS One 7:18
Belforte JE et al (2010) Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 13:76–83
Bjarnadottir M et al (2007) Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1 +/− knock-outs compared with wild-type mice. J Neurosci 27:4519–4529. doi:10.1523/JNEUROSCI.4314-06.2007
Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J (2008) Sensory gating revisited: relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophr Res 99:238–249. doi:10.1016/j.schres.2007.10.034
Cardin JA et al (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459:663–667
Carlen M et al (2012) A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 17:537–548
Carlson GC et al (2011) Dysbindin-1 mutant mice implicate reduced fast-phasic inhibition as a final common disease mechanism in schizophrenia. Proc Natl Acad Sci USA 108:3
Chance FS, Abbott L, Reyes AD (2002) Gain modulation from background synaptic input Neuron 35:773–782
Deakin IH et al (2012) Transgenic overexpression of the type I isoform of neuregulin 1 affects working memory and hippocampal oscillations but not long-term potentiation. Cereb Cortex 22:1520–1529
Del Pino I et al (2013) Erbb4 deletion from fast-spiking interneurons causes schizophrenia-like phenotypes. Neuron 79:1152–1168
Destexhe A, Pare D (1999) Impact of network activity on the integrative properties of neocortical pyramidal neurons in vivo. J Neurophysiol 81:1531–1547
Detsch O, Vahle-Hinz C, Kochs E, Siemers M, Bromm B (1999) Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res 829:77–89
Detsch O, Kochs E, Siemers M, Bromm B, Vahle-Hinz C (2002a) Differential effects of isoflurane on excitatory and inhibitory synaptic inputs to thalamic neurones in vivo. Br J Anaesth 89:294–300
Detsch O, Kochs E, Siemers M, Bromm B, Vahle-Hinz C (2002b) Increased responsiveness of cortical neurons in contrast to thalamic neurons during isoflurane-induced EEG bursts in rats. Neurosci Lett 317:9–12
Duffy L, Cappas E, Scimone A, Schofield PR, Karl T (2008) Behavioral profile of a heterozygous mutant mouse model for EGF-like domain neuregulin 1. Behav Neurosci 122:748–759
Edgar JC et al (2013) Cortical thickness as a contributor to abnormal oscillations in schizophrenia? Neuroimage Clin 4:122–129
Ehrlichman RS et al (2009) Neuregulin 1 transgenic mice display reduced mismatch negativity, contextual fear conditioning and social interactions. Brain Res 1294:116–127. doi:10.1016/j.brainres.2009.07.065
Ewert TA, Vahle-Hinz C, Engel AK (2008) High-frequency whisker vibration is encoded by phase-locked responses of neurons in the rat’s barrel cortex. J Neurosci 28:5359–5368. doi:10.1523/JNEUROSCI.0089-08.2008
Fazzari P et al (2010) Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature 464:1376–1380
Featherstone RE, MT-L V, Suh JD, Lin R, Lucki I, Siegel SJ (2013) Electrophysiological and behavioral responses to ketamine in mice with reduced Akt1 expression. Psychopharmacology 227:639–649
Fisahn A, Neddens J, Yan L, Buonanno A (2009) Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex 19:612–618
Flynn G et al (2008) Increased absolute magnitude of gamma synchrony in first-episode psychosis. Schizophr Res 105:262–271
Gandal MJ, Edgar JC, Klook K, Siegel SJ (2012a) Gamma synchrony: towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology 62:1504–1518
Gandal MJ et al (2012b) GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction Transl. Psychiatry 17:69
Gratton A, Hoffer BJ, Freedman R (1987) Electrophysiological effects of phencyclidine in the medial prefrontal cortex of the rat. Neuropharmacology 26:1275–1283
Greenwood TA et al (2011) Analysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia AJ. Psychiatry 168:930–946
Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL (2012) Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One 7:13
Hakami T et al (2009) NMDA receptor hypofunction leads to generalized and persistent aberrant gamma oscillations independent of hyperlocomotion and the state of consciousness PLoS One 4:0006755
Hirose N, Yoshida Y, Koide S, Takada K, Saigusa T, Koshikawa N (1998) Effects of propofol and fentanyl on extracellular levels of gamma-aminobutyric acid in the rat nucleus accumbens: an in vivo microdialysis study. J Oral Sci 40:165–170
Ho N, Destexhe A (2000) Synaptic background activity enhances the responsiveness of neocortical pyramidal neurons. J Neurophysiol 84:1488–1496
Homayoun H, Moghaddam B (2007) NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci 27:11496–11500
Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA, Lahti AC (2010) Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology 35:632–640
Horita A, Carino MA, Chinn C (1989) Fentanyl produces cholinergically-mediated analeptic and EEG arousal effects in rats. Neuropharmacology 28:481–486. doi:10.1016/0028-3908(89)90083-X
Huang YZ et al (2000) Regulation of Neuregulin Signaling by PSD-95 Interacting with ErbB4 at CNS Synapses. Neuron 26:443–455. doi:10.1016/S0896-6273(00)81176-9
Hume AL, Durkin MA (1986) Central and spinal somatosensory conduction times during hypothermic cardiopulmonary bypass and some observations on the effects of fentanyl and isoflurane anesthesia. Electroencephal Clin Neurophysiol Evoked Potential Sect 65:46–58. doi:10.1016/0168-5597(86)90036-5
Ikuta T et al (2007) Differences in waveforms of cerebral evoked potentials among healthy subjects, schizophrenics, manic-depressives and epileptics. J Med Invest 54:303–315
Itil TM, Saletu B, Davis S (1972) EEG findings in chronic schizophrenics based on digital computer period analysis and analog power spectra. Biol Psychiatry 5:1–13
Jackson ME, Homayoun H, Moghaddam B (2004) NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc Natl Acad Sci USA 101:8467–8472
Jin TE, Witzemann V, Brecht M (2004) Fiber types of the intrinsic whisker muscle and whisking behavior. J Neurosci 24:3386–3393
Kikuchi M et al (2011) Frontal areas contribute to reduced global coordination of resting-state gamma activities in drug-naive patients with schizophrenia. Schizophr Res 130:187–194
Kittelberger K, Hur EE, Sazegar S, Keshavan V, Kocsis B (2012) Comparison of the effects of acute and chronic administration of ketamine on hippocampal oscillations: relevance for the NMDA receptor hypofunction model of schizophrenia. Brain Struct Funct 217:395–409
Kocsis B (2012) Differential Role of NR2A and NR2B Subunits in N-Methyl-D-Aspartate Receptor Antagonist-Induced Aberrant Cortical Gamma Oscillations. Biol Psychiatry 71:987–995. doi:10.1016/j.biopsych.2011.10.002
Kouvaras E et al (2008) Fentanyl treatment reduces GABAergic inhibition in the CA1 area of the hippocampus 24 h after acute exposure to the drug. Neuropharmacology 55:1172–1182. doi:10.1016/j.neuropharm.2008.07.025
Krishnan GP, Vohs JL, Hetrick WP, Carroll CA, Shekhar A, Bockbrader MA, O’Donnell BF (2005) Steady state visual evoked potential abnormalities in schizophrenia. Clin Neurophysiol 116:614–624
Krishnan G, Hetrick W, Brenner C, Shekhar A, Steffen A, O’Donnell B (2009) Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage 47:1711
Kulikova SP, Tolmacheva EA, Anderson P, Gaudias J, Adams BE, Zheng T, Pinault D (2012) Opposite effects of ketamine and deep brain stimulation on rat thalamocortical information processing. Eur J Neurosci 36:3407–3419. doi:10.1111/j.1460-9568.2012.08263.x
Kwon JS et al (1999) Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 56:1001–1005
Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A (2005) Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci 25:9378–9383
Li D, Collier DA, He L (2006) Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet 15:1995–2002. doi:10.1093/hmg/ddl122
Li KX et al (2011) Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat Neurosci 15:267–273
Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL (2006) Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry 60:1231–1240
Lodge DJ, Behrens MM, Grace AA (2009) A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci 29:2344–2354
Ma J, Leung LS (2007) The supramammillo-septal-hippocampal pathway mediates sensorimotor gating impairment and hyperlocomotion induced by MK-801 and ketamine in rats. Psychopharmacology 191:961–974
Marquis KL, Paquette NC, Gussio RP, Moreton JE (1989) Comparative electroencephalographic and behavioral effects of phencyclidine, (+)-SKF-10,047 and MK-801 in rats. J Pharmacol Exp Ther 251:1104–1112
Mei L, Xiong WC (2008) Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 9:437–452. doi:10.1038/nrn2392
Meyer D, Birchmeier C (1995) Multiple essential functions of neuregulin in development. Nature 378:386–390
Meyer D, Yamaai T, Garratt A, Riethmacher-Sonnenberg E, Kane D, Theill LE, Birchmeier C (1997) Isoform-specific expression and function of neuregulin. Development 124:3575–3586
Molina LA, Skelin I, Gruber AJ (2014) Acute NMDA receptor antagonism disrupts synchronization of action potential firing in rat prefrontal cortex. PLoS One 9
Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM (2011) Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol 79:55–63
Munafo MR, Thiselton DL, Clark TG, Flint J (2006) Association of the NRG1 gene and schizophrenia: a meta-analysis. Mol Psychiatry 11:539–546. doi:10.1038/sj.mp.4001817
Murray JD, Anticevic A, Gancsos M, Ichinose M, Corlett PR, Krystal JH, Wang XJ (2012) Linking Microcircuit Dysfunction to Cognitive Impairment: effects of disinhibition associated with Schizophrenia in a cortical working memory model. Cereb Cortex 29:29
Nason MW Jr, Adhikari A, Bozinoski M, Gordon JA, Role LW (2011) Disrupted activity in the hippocampal-accumbens circuit of type III neuregulin 1 mutant mice. Neuropsychopharmacology 36:488–496. doi:10.1038/npp.2010.180
Newell KA, Karl T, Huang XF (2013) A neuregulin 1 transmembrane domain mutation causes imbalanced glutamatergic and dopaminergic receptor expression in mice. Neuroscience 248:670–680
Nicolás MJ, López-Azcárate J, Valencia M, Alegre M, Pérez-Alcázar M, Iriarte J, Artieda J (2011) Ketamine-Induced Oscillations in the Motor Circuit of the Rat Basal Ganglia. PLoS One 6:e21814. doi:10.1371/journal.pone.0021814
Pinault D (2008) N-methyl D-aspartate receptor antagonists ketamine and MK-801 induce wake-related aberrant gamma oscillations in the rat neocortex. Biol Psychiatry 63:730–735
Ritt JT, Andermann ML, Moore CI (2008) Embodied information processing: vibrissa mechanics and texture features shape micromotions in actively sensing rats. Neuron 57:599–613. doi:10.1016/j.neuron.2007.12.024
Saunders JA, Gandal MJ, Siegel SJ (2012) NMDA antagonists recreate signal-to-noise ratio and timing perturbations present in schizophrenia. Neurobiol Dis 46:93–100
Shagass C (1977) Early evoked potentials. Schizophr Bull 3:80–92
Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA (2010) Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464:763–767
Sohal VS, Zhang F, Yizhar O, Deisseroth K (2009) Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459:698–702
Spencer KM (2012) Baseline gamma power during auditory steady-state stimulation in schizophrenia. Front Hum Neurosci 5
Spencer KM, Salisbury DF, Shenton ME, McCarley RW (2008) Gamma-band auditory steady-state responses are impaired in first episode psychosis. Biol Psychiatry 64:369–375
Sun J, Kuo PH, Riley BP, Kendler KS, Zhao Z (2008) Candidate genes for schizophrenia: a survey of association studies and gene ranking. Am J Med Genet B Neuropsychiatr Genet 5:1173–1181
Teale P, Pasko B, Collins D, Rojas D, Reite M (2013) Somatosensory timing deficits in schizophrenia. Psychiatry Res 212:73–78
Ting AK et al (2011) Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci 31:15–25
Tsuchimoto R et al (2011) Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr Res 133:99–105
Vahle-Hinz C, Detsch O, Siemers M, Kochs E (2007) Contributions of GABAergic and glutamatergic mechanisms to isoflurane-induced suppression of thalamic somatosensory information transfer. Exp Brain Res 176:159–172
Venables NC, Bernat EM, Sponheim SR (2009) Genetic and disorder-specific aspects of resting state EEG abnormalities in schizophrenia. Schizophr Bull 35:826–839
Vogels TP, Abbott LF (2007) Gating deficits in model networks: a path to schizophrenia? Pharmacopsychiatry 40:S73–S77
Vohs JL, Chambers RA, O’Donnell BF, Krishnan GP, Morzorati SL (2012) Auditory steady state responses in a schizophrenia rat model probed by excitatory/inhibitory receptor manipulation. Int J Psychophysiol 86:136–142
Voigts J, Sakmann B, Celikel T (2008) Unsupervised whisker tracking in unrestrained behaving animals. J Neurophysiol 100:504–515
Volman V, Behrens MM, Sejnowski TJ (2011) Downregulation of parvalbumin at cortical GABA synapses reduces network gamma oscillatory activity. J Neurosci 31:18137–18148
Winterer G, Weinberger DR (2004) Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci 27:683–690. doi:10.1016/j.tins.2004.08.002
Winterer G et al (2000) Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol 111:837–849
Winterer G, Coppola R, Goldberg TE, Egan MF, Jones DW, Sanchez CE, Weinberger DR (2004) Prefrontal broadband noise, working memory, and genetic risk for schizophrenia AJ. Psychiatry 161:490–500
Wood J, Kim Y, Moghaddam B (2012) Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci 32:3022–3031
Yoshida Y, Koide S, Hirose N, Takada K, Tomiyama K, Koshikawa N, Cools AR (1999) Fentanyl increases dopamine release in rat nucleus accumbens: involvement of mesolimbic mu- and delta-2-opioid receptors. Neuroscience 92:1357–1365
Zhang Y, Yoshida T, Katz DB, Lisman JE (2012) NMDAR antagonist action in thalamus imposes delta oscillations on the hippocampus. J Neurophysiol 107:3181–3189
Acknowledgments
We would like to thank Steve Siegel and Ted Brodkin (University of Pennsylvania) for kindly providing NRG1 (+/−) mutants and WT mice. This work was supported by the IRTG 1328 Schizophrenia and Autism of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; C. Barz), the Interdisciplinary Center for Clinical Research (IZKF Aachen) within the Faculty of Medicine at the RWTH Aachen University (C. Barz), the postdoctoral fellowship of the Fondation pour la Recherche Medicale SPE20070709864 (T. Bessaih), the Barrel Cortex Function (BaCoFun) research group of the DFG (D. Feldmeyer), the Helmholtz association (D. Feldmeyer), as well as the National Institute of Health (NIH) grants MH064045 (T. Abel), P50-MH096891 (T. Abel and R. Gur, PI) and R01 EY020765 (D. Contreras).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barz, C.S., Bessaih, T., Abel, T. et al. Sensory encoding in Neuregulin 1 mutants. Brain Struct Funct 221, 1067–1081 (2016). https://doi.org/10.1007/s00429-014-0955-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0955-x