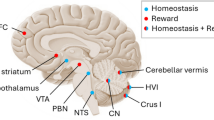
Abstract
Lower brainstem projections from nucleus accumbens (Ac) subregions to the parabrachial complex (PB), the nucleus of the solitary tract and the vagal motor nuclei have been described previously in the domestic chick by our group. Such projections, particulary those from the core and rostral pole regions of Ac have not been found in mammals or pigeons. Here we report on the presence of neurotensin (NT) in the neurons projecting from different Ac subnuclei, and also from the bed nucleus of stria terminalis, to the PB in the domestic chicken. The study is based upon correlated retrograde tracing (using Fast Blue) and NT immunohistochemistry, supplemented with regional charting and quantitative analysis of double-labeled neurons. The number of retrogradely labeled cells in Ac subdivisions reflects the size of FB tracer deposit, and the degree to which it extends to the medial PB. Of all Ac subregions, the core contained the largest amount of double-labeled cells. The findings demonstrate that the anatomical pathway through which the Ac can directly modulate taste-responsive neurons of the PB employs mainly neurotensin as a neuromodulator. The observed anatomical difference between mammals and birds is either a general taxonomic feature or it reflects feeding strategies specific for the domestic chick. The results are also relevant to a better understanding of the role of NT in food intake and reward-related behaviors in birds.







Similar content being viewed by others
References
Alexander MJ, Miller MA, Dorsa DM, Bullock BP, Melloni RH Jr, Dobner PR, Leeman SE (1989) Distribution of neurotensin/neuromedin N mRNA in rat forebrain: unexpected abundance in hippocampus and subiculum. Proc Natl Acad Sci USA 86:5202–5206
Arends JJ, Wild JM, Zeigler HP (1988) Projections of the nucleus of the tractus solitarius in the pigeon (Columba livia). J Comp Neurol 278:405–429. doi:10.1002/cne.902780310
Atoji Y, Watanabe H, Nimamoto N, Sugiyama M, Yamamoto Y, Suzuki Y (1994) Neurotensin immunoreactive cells in the gastrointestinal epithelium of the chicken, pigeon and Japanese quail. Eur J Histochem 38:65–72
Atoji Y, Hirasawa Y, Yamamoto Y, Suzuki Y (1995a) Distribution of neurotensin-immunoreactive neurons in the digestive tract of the chicken. J Auton Nerv Syst 53:185–194
Atoji Y, Watanabe H, Yamamoto Y, Suzuki Y (1995b) Distribution of neurotensin-containing neurons in the central nervous system of the dog. J Comp Neurol 353:67–88. doi:10.1002/cne.903530108
Atoji Y, Shibata N, Yamamoto Y, Suzuki Y (1996) Distribution of neurotensin-containing neurons in the central nervous system of the pigeon and the chicken. J Comp Neurol 375:187–211. doi:10.1002/(SICI)1096-9861(19961111)375:2<187:AID-CNE2>3.0.CO;2-Z
Balint E, Csillag A (2007) Nucleus accumbens subregions: hodological and immunohistochemical study in the domestic chick (Gallus domesticus). Cell Tissue Res 327:221–230. doi:10.1007/s00441-006-0295-0
Balint E, Mezey S, Csillag A (2011) Efferent connections of nucleus accumbens subdivisions of the domestic chicken (Gallus domesticus): an anterograde pathway tracing study. J Comp Neurol 519:2922–2953. doi:10.1002/cne.22672
Berk ML, Smith SE, Mullins LA (1993) Distribution, parabrachial region projection, and coexistence of neuropeptide and catecholamine cells of the nucleus of the solitary tract in the pigeon. J Comp Neurol 327:416–441. doi:10.1002/cne.903270308
Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28:309–369
Berridge KC, Robinson TE, Aldridge JW (2009) Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9:65–73. doi:10.1016/j.coph.2008.12.014
Carlezon WA Jr, Thomas MJ (2009) Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56(Suppl 1):122–132. doi:10.1016/j.neuropharm.2008.06.075
Carraway R, Bhatnagar YM (1980) Immunochemical characterization of neurotensin-like peptides in chicken. Peptides 1:159–165
Carraway RE, Mitra SP, Duke GE (1993a) A common precursor to neurotensin and LANT6 and its differential processing in chicken tissues. Peptides 14:1245–1251
Carraway RE, Mitra SP, Joyce TJ (1993b) Tissue-specific processing of neurotensin/neuromedin-N precursor in cat. Regul Pept 43:97–106
Cho YK, Li CS, Smith DV (2003) Descending influences from the lateral hypothalamus and amygdala converge onto medullary taste neurons. Chem Senses 28:155–171
Clarke JC, Westbrook RF, Irwin J (1979) Potentiation instead of overshadowing in the pigeon. Behav Neural Biol 25:18–29
Corbit LH, Muir JL, Balleine BW (2001) The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci 21:3251–3260
Csillag A (1999) Striato-telencephalic and striato-tegmental circuits: relevance to learning in domestic chicks. Behav Brain Res 98:227–236
Di Chiara G (2002) Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137:75–114
Dobner PR, Barber DL, Villa-Komaroff L, McKiernan C (1987) Cloning and sequence analysis of cDNA for the canine neurotensin/neuromedin N precursor. Proc Natl Acad Sci USA 84:3516–3520
Esposito V, De Girolamo P, Gargiulo G (1997) Neurotensin-like immunoreactivity in the brain of the chicken, Gallus domesticus. J Anat 191:537–546. doi:10.1046/j.1469-7580.1997.19140537.x
Franchina JJ (1997) Effects of taste preexposure on aversion training to visual cues in chicks (Gallus domesticus). Physiol Behav 62:611–615
Franchina JJ, Moon C, Peters S (1997) Effects of toxin magnitude on taste aversion and taste-potentiated aversion to visual cues in chicks (Gallus domesticus). Physiol Behav 62:605–609
Garcia J, Kimeldorf DJ, Koelling RA (1955) Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 122:157–158
Gillette K, Martin GM, Bellingham WP (1980) Differential use of food and water cues in the formation of conditioned aversions by domestic chicks (Gallus gallus). J Exp Psychol Anim Behav Process 6:99–111
Goedert M, Schwartz WN, Williams BJ (1985) The comparative distribution of [Lys8-Asn9]-neurotensin8-13-like immunoreactivity in chicken and rat tissues. Brain Res 342:259–265
Grill HJ (1985) Introduction: physiological mechanisms in conditioned taste aversions. Ann N Y Acad Sci 443:67–88
Groenewegen HJ, Russchen FT (1984) Organization of the efferent projections of the nucleus accumbens to pallidal, hypothalamic, and mesencephalic structures: a tracing and immunohistochemical study in the cat. J Comp Neurol 223:347–367. doi:10.1002/cne.902230303
Groenewegen HJ, Uylings HB (2000) The prefrontal cortex and the integration of sensory, limbic and autonomic information. Prog Brain Res 126:3–28. doi:10.1016/S0079-6123(00)26003-2
Groenewegen HJ, Wright CI, Beijer AV, Voorn P (1999) Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877:49–63
Hajnal A, Smith GP, Norgren R (2004) Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol 286:R31–R37. doi:10.1152/ajpregu.00282.2003
Hajnal A, Norgren R, Kovacs P (2009) Parabrachial coding of sapid sucrose: relevance to reward and obesity. Ann N Y Acad Sci 1170:347–364. doi:10.1111/j.1749-6632.2009.03930.x
Hanics J, Balint E, Milanovich D, Zachar G, Adam A, Csillag A (2012) Amygdalofugal axon terminals immunoreactive for l-aspartate or l-glutamate in the nucleus accumbens of rats and domestic chickens: a comparative electron microscopic immunocytochemical study combined with anterograde pathway tracing. Cell Tissue Res 350:409–423. doi:10.1007/s00441-012-1494-5
Hayne H, Rovee-Collier C, Collier G, Tudor L, Morgan CA (1996) Learning and retention of conditioned aversions by freely feeding chicks. Dev Psychobiol 29:417–431
Husband SA, Shimizu T (2011) Calcium-binding protein distributions and fiber connections of the nucleus accumbens in the pigeon (Columba livia). J Comp Neurol 519:1371–1394. doi:10.1002/cne.22575
Jennes L, Stumpf WE, Kalivas PW (1982) Neurotensin: topographical distribution in rat brain by immunohistochemistry. J Comp Neurol 210:211–224. doi:10.1002/cne.902100302
Kelley AE (1999) Neural integrative activities of nucleus accumbens subregions in relation to learning and motivation. Psychobiology 27:198–213
Kelley AE (2004) Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27:765–776. doi:10.1016/j.neubiorev.2003.11.015
Kuenzel WJ, Masson M (1988) A stereotaxic atlas of the brain of the chick (Gallus domesticus). Johns Hopkins University Press, Baltimore
Li CS, Cho YK (2006) Efferent projection from the bed nucleus of the stria terminalis suppresses activity of taste-responsive neurons in the hamster parabrachial nuclei. Am J Physiol Regul Integr Comp Physiol 291:R914–R926. doi:10.1152/ajpregu.00750.2005
Li CS, Cho YK, Smith DV (2002) Taste responses of neurons in the hamster solitary nucleus are modulated by the central nucleus of the amygdala. J Neurophysiol 88:2979–2992. doi:10.1152/jn.00239.2002
Li CS, Cho YK, Smith DV (2005) Modulation of parabrachial taste neurons by electrical and chemical stimulation of the lateral hypothalamus and amygdala. J Neurophysiol 93:1183–1196. doi:10.1152/jn.00828.2004
Li CS, Chung S, Lu DP, Cho YK (2012) Descending projections from the nucleus accumbens shell suppress activity of taste-responsive neurons in the hamster parabrachial nuclei. J Neurophysiol 108:1288–1298. doi:10.1152/jn.00121.2012
Loriaux AL, Roitman JD, Roitman MF (2011) Nucleus accumbens shell, but not core, tracks motivational value of salt. J Neurophysiol 106:1537–1544. doi:10.1152/jn.00153.2011
Lundy RF Jr, Norgren R (2001) Pontine gustatory activity is altered by electrical stimulation in the central nucleus of the amygdala. J Neurophysiol 85:770–783
Lundy R, Norgren R (2004a) Gustatory system. In: Paxinos G (ed) The rat nervous system, 3rd edn. Elsevier Academic Press, San Diego, pp 891–921
Lundy RF Jr, Norgren R (2004b) Activity in the hypothalamus, amygdala, and cortex generates bilateral and convergent modulation of pontine gustatory neurons. J Neurophysiol 91:1143–1157. doi:10.1152/jn.00840.2003
McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF (2012) Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci 6:137. doi:10.3389/fnins.2012.00137
Milner TA, Pickel VM (1986) Neurotensin in the rat parabrachial region: ultrastructural localization and extrinsic sources of immunoreactivity. J Comp Neurol 247:326–343. doi:10.1002/cne.902470304
Moga MM, Saper CB, Gray TS (1990) Neuropeptide organization of the hypothalamic projection to the parabrachial nucleus in the rat. J Comp Neurol 295:662–682. doi:10.1002/cne.902950409
Mungarndee SS, Lundy RF Jr, Norgren R (2008) Expression of Fos during sham sucrose intake in rats with central gustatory lesions. Am J Physiol Regul Integr Comp Physiol 295:R751–R763. doi:10.1152/ajpregu.90344.2008
Norgren R, Hajnal A, Mungarndee SS (2006) Gustatory reward and the nucleus accumbens. Physiol Behav 89:531–535. doi:10.1016/j.physbeh.2006.05.024
Olson BJ, Markwell J (2007) Assays for determination of protein concentration. Curr Protoc Protein Sci 3:34. doi:10.1002/0471140864.ps0304s48
Patterson TA, Rose SP (1992) Memory in the chick: multiple cues, distinct brain locations. Behav Neurosci 106:465–470
Puelles L, Martinez-de-la-Torre M, Paxinos G, Watson C, Martinez S (2007) The chick brain in stereotaxic coordinates: an atlas correlating avian and mammalian neuroanatomy. Academic Press, San Diego
Ramirez-Lugo L, Zavala-Vega S, Bermudez-Rattoni F (2006) NMDA and muscarinic receptors of the nucleus accumbens have differential effects on taste memory formation. Learn Mem 13:45–51. doi:10.1101/lm.103206
Ramirez-Lugo L, Nunez-Jaramillo L, Bermudez-Rattoni F (2007) Taste memory formation: role of nucleus accumbens. Chem Senses 32:93–97. doi:10.1093/chemse/bjl023
Reinecke M (1985) Neurotensin. Immunohistochemical localization in central and peripheral nervous system and in endocrine cells and its functional role as neurotransmitter and endocrine hormone. Prog Histochem Cytochem 16:1–172
Reiner A, Anderson KD (1993) Co-occurrence of gamma-aminobutyric acid, parvalbumin and the neurotensin-related neuropeptide LANT6 in pallidal, nigral and striatal neurons in pigeons and monkeys. Brain Res 624:317–325
Roitman MF, Wheeler RA, Tiesinga PH, Roitman JD, Carelli RM (2010) Hedonic and nucleus accumbens neural responses to a natural reward are regulated by aversive conditioning. Learn Mem 17:539–546. doi:10.1101/lm.1869710
Rose SP (2000) God’s organism? The chick as a model system for memory studies. Learn Mem 7:1–17
Saggu S, Lundy RF (2008) Forebrain neurons that project to the gustatory parabrachial nucleus in rat lack glutamic acid decarboxylase. Am J Physiol Regul Integr Comp Physiol 294:R52–R57. doi:10.1152/ajpregu.00635.2007
Saleh TM, Cechetto DF (1993) Peptides in the parabrachial nucleus modulate visceral input to the thalamus. Am J Physiol 264:R668–R675
Saleh TM, Cechetto DF (1994) Neurotransmitters in the parabrachial nucleus mediating visceral input to the thalamus in rats. Am J Physiol 266:R1287–R1296
Saleh TM, Cechetto DF (1995) Neurochemical interactions in the parabrachial nucleus mediating visceral inputs to visceral thalamic neurons. Am J Physiol 268:R786–R795
Saleh TM, Kombian SB, Zidichouski JA, Pittman QJ (1997) Cholecystokinin and neurotensin inversely modulate excitatory synaptic transmission in the parabrachial nucleus in vitro. Neuroscience 77:23–35
Shimura T, Tanaka H, Yamamoto T (1997) Salient responsiveness of parabrachial neurons to the conditioned stimulus after the acquisition of taste aversion learning in rats. Neuroscience 81:239–247
Stewart MG, Rusakov DA (1995) Morphological changes associated with stages of memory formation in the chick following passive avoidance training. Behav Brain Res 66:21–28
Stratford TR, Kelley AE (1999) Evidence of a functional relationship between the nucleus accumbens shell and lateral hypothalamus subserving the control of feeding behavior. J Neurosci 19:11040–11048
Sugimoto T, Mizuno N (1987) Neurotensin in projection neurons of the striatum and nucleus accumbens, with reference to coexistence with enkephalin and GABA: an immunohistochemical study in the cat. J Comp Neurol 257:383–395. doi:10.1002/cne.902570307
Troiano R, Siegel A (1978) Efferent connections of the basal forebrain in the cat: the nucleus accumbens. Exp Neurol 61:185–197
Usuda I, Tanaka K, Chiba T (1998) Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res 797:73–93
Vincent JP, Mazella J, Kitabgi P (1999) Neurotensin and neurotensin receptors. Trends Pharmacol Sci 20:302–309
Wild JM, Arends JJA (1987) A respiratory-vocal pathway in the brain-stem of the pigeon. Brain Res 407:191–194
Wild JM, Arends JJ, Zeigler HP (1990) Projections of the parabrachial nucleus in the pigeon (Columba livia). J Comp Neurol 293:499–523. doi:10.1002/cne.902930402
Yamamoto T et al (2009) Functional organization of the rodent parabrachial nucleus. Ann N Y Acad Sci 1170:378–382. doi:10.1111/j.1749-6632.2009.03883.x
Zahm DS (1987) Neurotensin-immunoreactive neurons in the ventral striatum of the adult rat: ventromedial caudate-putamen, nucleus accumbens and olfactory tubercle. Neurosci Lett 81:41–47
Zahm DS, Heimer L (1993) Specificity in the efferent projections of the nucleus accumbens in the rat: comparison of the rostral pole projection patterns with those of the core and shell. J Comp Neurol 327:220–232. doi:10.1002/cne.903270205
Zahm DS, Jensen SL, Williams ES, Martin JR 3rd (1999) Direct comparison of projections from the central amygdaloid region and nucleus accumbens shell. Eur J Neurosci 11:1119–1126
Acknowledgments
The authors wish to thank Dr. János Hanics for valuable technical suggestions. The study was supported by a Hungarian research Grant OTKA K-109077.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bálint, E., Balázsa, T., Zachar, G. et al. Neurotensin: revealing a novel neuromodulator circuit in the nucleus accumbens–parabrachial nucleus projection of the domestic chick. Brain Struct Funct 221, 605–616 (2016). https://doi.org/10.1007/s00429-014-0928-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0928-0