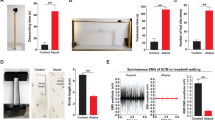
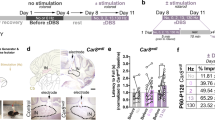
Abstract
Deep brain stimulation (DBS) is effective in managing motor symptoms of Parkinson’s disease in well-selected individuals. Recently, research has shown that DBS in the basal ganglia (BG) can alter neural circuits beyond the traditional basal ganglia-thalamus-cortical (BG-TH-CX) loop. For instance, functional imaging showed alterations in cerebellar activity with DBS in the subthalamic nucleus (STN). However, these imaging studies revealed very little about how cell-specific cerebellar activity responds to STN stimulation or if these changes contribute to its efficacy. In this study, we assess whether STN-DBS provides efficacy in managing motor symptoms in Parkinson’s disease by recruiting cerebellar activity. We do this by applying STN-DBS in hemiparkinsonian rats and simultaneously recording neuronal activity from the STN, brainstem and cerebellum. We found that STN neurons decreased spiking activity by 55 % during DBS (P = 0.038), which coincided with a decrease in most pedunculopontine tegmental nucleus and Purkinje neurons by 29 % (P < 0.001) and 28 % (P = 0.003), respectively. In contrast, spike activity in the deep cerebellar nuclei increased 45 % during DBS (P < 0.001), which was likely from reduced afferent activity of Purkinje cells. Then, we applied STN-DBS at sub-therapeutic current along with stimulation of the deep cerebellar nuclei and found similar improvement in forelimb akinesia as with therapeutic STN-DBS alone. This suggests that STN-DBS can engage cerebellar activity to improve parkinsonian motor symptoms. Our study is the first to describe how STN-DBS in Parkinson’s disease alters cerebellar activity using electrophysiology in vivo and reveal a potential for stimulating the cerebellum to potentiate deep brain stimulation of the subthalamic nucleus.






Similar content being viewed by others
References
Allen GI, Tsukahara N (1974) Cerebrocerebellar communication systems. Physiol Rev 54:957–1006
Allen GI, Azzena GB, Ohno T (1974) Somatotopically organized inputs from fore- and hindlimb areas of sensorimotor cortex to cerebellar Purkyne cells. Exp Brain Res 20:255–272
Aravamuthan BR, Muthusamy KA, Stein JF, Aziz TZ, Johansen-Berg H (2007) Topography of cortical and subcortical connections of the human pedunculopontine and subthalamic nuclei. NeuroImage 37:694–705. doi:10.1016/j.neuroimage.2007.05.050
Asanuma K et al (2006) Network modulation in the treatment of Parkinson’s disease. Brain 129:2667–2678 awl16210.1093/brain/awl162
Azulay JP, Mesure S, Amblard B, Blin O, Sangla I, Pouget J (1999) Visual control of locomotion in Parkinson’s disease. Brain 122(Pt 1):111–120
Ballanger B et al (2009) Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: a [(15)O] H2O PET study. Hum Brain Mapp 30:3901–3909. doi:10.1002/hbm.20815
Barmack NH, Yakhnitsa V (2011) Topsy turvy: functions of climbing and mossy fibers in the vestibulo-cerebellum. Neuroscientist 17:221–236. doi:10.1177/1073858410380251
Bastian AJ, Martin TA, Keating JG, Thach WT (1996) Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 76:492–509
Bonnefoi-Kyriacou B, Trouche E, Legallet E, Viallet F (1995) Planning and execution of pointing movements in cerebellar patients. Mov Disord 10:171–178. doi:10.1002/mds.870100207
Bostan AC, Strick PL (2010) The cerebellum and basal ganglia are interconnected. Neuropsychol Rev 20:261–270. doi:10.1007/s11065-010-9143-9
Bostan AC, Dum RP, Strick PL (2010) The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107:8452–8456. doi:10.1073/pnas.1000496107
Bradberry TJ et al (2011) Common and unique responses to dopamine agonist therapy and deep brain stimulation in Parkinson’s disease: an H(2)(15)O PET study. Brain Stimul 5:605–615. doi:10.1016/j.brs.2011.09.002
Braitenberg V, Heck D, Sultan F (1997) The detection and generation of sequences as a key to cerebellar function: experiments and theory. Behav Brain Sci 20:229–245. doi:10.1016/j.brs.2011.09.002 (discussion 245−277)
Breit S, Martin A, Lessmann L, Cerkez D, Gasser T, Schulz JB (2008) Bilateral changes in neuronal activity of the basal ganglia in the unilateral 6-hydroxydopamine rat model. J Neurosci Res 86:1388–1396. doi:10.1002/jnr.21588
Canedo A (1997) Primary motor cortex influences on the descending and ascending systems. Prog Neurobiol 51:287–335
Castrioto A, Lozano AM, Poon YY, Lang AE, Fallis M, Moro E (2011) Ten-year outcome of subthalamic stimulation in Parkinson disease: a blinded evaluation. Arch Neurol 68:1550–1556 archneurol.2011.182. doi:10.1001/archneurol.2011.182
Cerminara NL, Rawson JA, Apps R (2010) Electrophysiological characterization of the cerebellum in the arterially perfused hindbrain and upper body of the rat. Cerebellum 9:218–231. doi:10.1007/s12311-009-0152-2
Chuma T, Faruque Reza M, Ikoma K, Mano Y (2006) Motor learning of hands with auditory cue in patients with Parkinson’s disease. J Neural Transm 113:175–185. doi:10.1007/s00702-005-0314-4
Cicirata F, Angaut P, Serapide MF, Panto MR, Nicotra G (1992) Multiple representation in the nucleus lateralis of the cerebellum: an electrophysiologic study in the rat. Exp Brain Res 89:352–362
Cilia R et al (2009) Clinical and cerebral activity changes induced by subthalamic nucleus stimulation in advanced Parkinson’s disease: a prospective case-control study. Clin Neurol Neurosurg 111:140–146. doi:10.1016/j.clineuro.2008.09.018
Coltz JD, Johnson MT, Ebner TJ (1999) Cerebellar Purkinje cell simple spike discharge encodes movement velocity in primates during visuomotor arm tracking. J Neurosci 19:1782–1803
D’Angelo E et al (2011) The cerebellar network: from structure to function and dynamics. Brain Res Rev 66:5–15. doi:10.1016/j.brainresrev.2010.10.002
Do MT, Bean BP (2003) Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39:109–120
Doya K (2000) Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol 10:732–739. pii: S0959-4388(00)00153-7
Dum RP, Strick PL (2003) An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol 89:634–639. doi:10.1152/jn.00626.2002
Eccles JC, Sabah NH, Schmidt RF, Taborikova H (1972) Integration by Purkyne cells of mossy and climbing fiber inputs from cutaneous mechanoreceptors. Exp Brain Res 15:498–520
Erez Y, Tischler H, Moran A, Bar-Gad I (2010) Generalized framework for stimulus artifact removal. J Neurosci Methods 191:45–59. doi:10.1016/j.jneumeth.2010.06.005
Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB (2013) Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136:2405–2418. doi:10.1093/brain/awt172
Freeman JS, Cody FW, Schady W (1993) The influence of external timing cues upon the rhythm of voluntary movements in Parkinson’s disease. J Neurol Neurosurg Psychiatry 56:1078–1084
Fu QG, Flament D, Coltz JD, Ebner TJ (1997) Relationship of cerebellar Purkinje cell simple spike discharge to movement kinematics in the monkey. J Neurophysiol 78:478–491
Gallay MN, Jeanmonod D, Liu J, Morel A (2008) Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct 212:443–463. doi:10.1007/s00429-007-0170-0
Galliano E, Mazzarello P, D’Angelo E (2010) Discovery and rediscoveries of Golgi cells. J Physiol 588:3639–3655. doi:10.1113/jphysiol.2010.189605
Georgiou N, Iansek R, Bradshaw JL, Phillips JG, Mattingley JB, Bradshaw JA (1993) An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain 116(Pt 6):1575–1587
Grafton ST, Turner RS, Desmurget M, Bakay R, Delong M, Vitek J, Crutcher M (2006) Normalizing motor-related brain activity: subthalamic nucleus stimulation in Parkinson disease. Neurology 66:1192–1199. doi:10.1212/01.wnl.0000214237.58321.c3 66/8/1192
Grant G (1962) Spinal course and somatotopically localized termination of the spinocerebellar tracts. An experimental study in the cat. Acta Physiol Scand Suppl 56:1–61
Grimaldi G, Manto M (2012) Topography of cerebellar deficits in humans. Cerebellum 11:336–351. doi:10.1007/s12311-011-0247-4
Grodd W, Hulsmann E, Lotze M, Wildgruber D, Erb M (2001) Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp 13:55–73
Gubellini P, Eusebio A, Oueslati A, Melon C, Kerkerian-Le Goff L, Salin P (2006) Chronic high-frequency stimulation of the subthalamic nucleus and L-DOPA treatment in experimental parkinsonism: effects on motor behaviour and striatal glutamate transmission. Eur J Neurosci 24:1802–1814. doi:10.1111/j.1460-9568.2006.05047.x EJN5047
Hammond C, Rouzaire-Dubois B, Feger J, Jackson A, Crossman AR (1983) Anatomical and electrophysiological studies on the reciprocal projections between the subthalamic nucleus and nucleus tegmenti pedunculopontinus in the rat. Neuroscience 9:41–52. pii: S0306-4522(83)90045-3
Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I (2011) Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 69:269–281. doi:10.1002/ana.22361
Hilker R et al (2003) Deep brain stimulation of the subthalamic nucleus does not increase the striatal dopamine concentration in parkinsonian humans. Mov Disord 18:41–48. doi:10.1002/mds.10297
Hilker R et al (2004) Subthalamic nucleus stimulation restores glucose metabolism in associative and limbic cortices and in cerebellum: evidence from a FDG-PET study in advanced Parkinson’s disease. J Cereb Blood Flow Metab 24:7–16. doi:10.1097/01.WCB.0000092831.44769.09
Hill KK et al (2013) Cerebral blood flow responses to dorsal and ventral STN DBS correlate with gait and balance responses in Parkinson’s disease. Exp Neurol 241:105–112. doi:10.1016/j.expneurol.2012.12.003
Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL (2005) The cerebellum communicates with the basal ganglia. Nat Neurosci 8:1491–1493. doi:10.1038/nn1544
Hudson JL et al (1993) Correlation of apomorphine- and amphetamine-induced turning with nigrostriatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain Res 626:167–174. pii: S0006-8993(93)90576-9
Ichinohe N, Teng B, Kitai ST (2000) Morphological study of the tegmental pedunculopontine nucleus, substantia nigra and subthalamic nucleus, and their interconnections in rat organotypic culture. Anat Embryol 201:435–453
Ito M, Yoshida M, Obata K, Kawai N, Udo M (1970) Inhibitory control of intracerebellar nuclei by the Purkinje cell axons. Exp Brain Res 10:64–80
Jackson A, Crossman AR (1983) Nucleus tegmenti pedunculopontinus: efferent connections with special reference to the basal ganglia, studied in the rat by anterograde and retrograde transport of horseradish peroxidase. Neuroscience 10:725–765
Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118:913–933
Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ (2000) Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain 123(Pt 6):1216–1228
Jueptner M, Weiller C (1998) A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain 121(Pt 8):1437–1449
Jueptner M, Jenkins IH, Brooks DJ, Frackowiak RS, Passingham RE (1996) The sensory guidance of movement: a comparison of the cerebellum and basal ganglia. Exp Brain Res 112:462–474
Kitahama K, Denoroy L, Goldstein M, Jouvet M, Pearson J (1988) Immunohistochemistry of tyrosine hydroxylase and phenylethanolamine N-methyltransferase in the human brain stem: description of adrenergic perikarya and characterization of longitudinal catecholaminergic pathways. Neuroscience 25:97–111
Kunzle H, Lotter G (1996) Efferents from the lateral frontal cortex to spinomedullary target areas, trigeminal nuclei, and spinally projecting brainstem regions in the hedgehog tenrec. J Comp Neurol 372:88–110. doi:10.1002/(SICI)1096-9861(19960812)372:1<88:AID-CNE7>3.0.CO;2-I
Lavoie B, Parent A (1994) Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J Comp Neurol 344:190–209. doi:10.1002/cne.903440203
LeDoux MS, Hurst DC, Lorden JF (1998) Single-unit activity of cerebellar nuclear cells in the awake genetically dystonic rat. Neuroscience 86:533–545
Lewis MM et al (2007) Task specific influences of Parkinson’s disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience 147:224–235. doi:10.1016/j.neuroscience.2007.04.006
Lewis MM, Galley S, Johnson S, Stevenson J, Huang X, McKeown MJ (2013) The role of the cerebellum in the pathophysiology of Parkinson’s disease. Can J Neurol Sci 40:299–306 P75M6432U3U148L5
Llinas R, Sugimori M (1980a) Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol 305:197–213
Llinas R, Sugimori M (1980b) Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol 305:171–195
Long-Smith CM, Sullivan AM, Nolan YM (2009) The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol 89:277–287. doi:10.1016/j.pneurobio.2009.08.001
Lozano AM, Lipsman N (2013) Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron 77:406–424. doi:10.1016/j.neuron.2013.01.020
Machado AG, Baker KB, Schuster D, Butler RS, Rezai A (2009) Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res 1280:107–116. doi:10.1016/j.brainres.2009.05.007
Machado AG, Cooperrider J, Furmaga HT, Baker KB, Park HJ, Chengabigabi Z, Gale J (2013) Chronic 30 Hz deep cerebellar stimulation coupled with training enhances post-ischemia motor recovery and peri-infarct synaptophysin expression in rodents. Neurosurgery 73:344–353. doi:10.1227/01.neu.0000430766.80102.ac
Manto M, Oulad Ben Taib N (2013) The contributions of the cerebellum in sensorimotor control: what are the prevailing opinions which will guide forthcoming studies? Cerebellum 12:313–315. doi:10.1007/s12311-013-0449-z
Martin GF, Cabana T, Culberson JL, Curry JJ, Tschismadia I (1980) The early development of corticobulbar and corticospinal systems. Studies using the North American opossum. Anat Embryol 161:197–213
Martinez-Gonzalez C, Bolam JP, Mena-Segovia J (2011) Topographical organization of the pedunculopontine nucleus. Front Neuroanat 5:22. doi:10.3389/fnana.2011.00022
Martinez-Gonzalez C, Wang HL, Micklem BR, Bolam JP, Mena-Segovia J (2012) Subpopulations of cholinergic, GABAergic and glutamatergic neurons in the pedunculopontine nucleus contain calcium-binding proteins and are heterogeneously distributed. Eur J Neurosci 35:723–734. doi:10.1111/j.1460-9568.2012.08002.x
McIntyre CC, Hahn PJ (2010) Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis 38:329–337. doi:10.1016/j.nbd.2009.09.022
Mehta A, Menalled L, Chesselet MF (2005) Behavioral responses to injections of muscimol into the subthalamic nucleus: temporal changes after nigrostriatal lesions. Neuroscience 131:769–778
Miall RC, Keating JG, Malkmus M, Thach WT (1998) Simple spike activity predicts occurrence of complex spikes in cerebellar Purkinje cells. Nat Neurosci 1:13–15. doi:10.1038/212
Moers-Hornikx VM, Vles JS, Tan SK, Cox K, Hoogland G, Steinbusch WM, Temel Y (2011) Cerebellar nuclei are activated by high-frequency stimulation of the subthalamic nucleus. Neurosci Lett 496:111–115. doi:10.1016/j.neulet.2011.03.094
Monakow KH, Akert K, Kunzle H (1979) Projections of precentral and premotor cortex to the red nucleus and other midbrain areas in Macaca fascicularis. Exp Brain Res 34:91–105
Moroz VM, Bures J (1984) Effects of lateralized reaching and cerebellar stimulation on unit activity of motor cortex and caudate nucleus in rats. Exp Neurol 84:47–57 0014-4886(84)90005-0
Mure H, Tang CC, Argyelan M, Ghilardi MF, Kaplitt MG, Dhawan V, Eidelberg D (2012) Improved sequence learning with subthalamic nucleus deep brain stimulation: evidence for treatment-specific network modulation. J Neurosci 32:2804–2813. doi:10.1523/JNEUROSCI.4331-11.2012 32/8/2804
Mushiake H, Strick PL (1995) Pallidal neuron activity during sequential arm movements. J Neurophysiol 74:2754–2758
Nauta HJ, Cole M (1974) Efferent projections of the subthalamic nucleus. Trans Am Neurol Assoc 99:170–173
Nelson MJ, Pouget P (2010) Do electrode properties create a problem in interpreting local field potential recordings? J Neurophysiol 103:2315–2317. doi:10.1152/jn.00157.2010
Newman DB, Ginsberg CY (1992) Brainstem reticular nuclei that project to the cerebellum in rats: a retrograde tracer study. Brain Behav Evol 39:24–68
Nowak DA, Tisch S, Hariz M, Limousin P, Topka H, Rothwell JC (2006) Sensory timing cues improve akinesia of grasping movements in Parkinson’s disease: a comparison to the effects of subthalamic nucleus stimulation. Mov Disord 21:166–172. doi:10.1002/mds.20657
Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, Foote KD (2005) Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol 62:1250–1255. doi:10.1001/archneur.62.8.noc40425
Olsson M, Nikkhah G, Bentlage C, Bjorklund A (1995) Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci 15:3863–3875
Parent A, Smith Y (1987) Organization of efferent projections of the subthalamic nucleus in the squirrel monkey as revealed by retrograde labeling methods. Brain Res 436:296–310 0006-8993(87)91674-X
Paxonis G, Watson C (1998) The rat atlas: in stereotaxic coordinates, 4th edn. Academic Press, San Diego
Peeters RR, Verhoye M, Vos BP, Van Dyck D, Van Der Linden A, De Schutter E (1999) A patchy horizontal organization of the somatosensory activation of the rat cerebellum demonstrated by functional MRI. Eur J Neurosci 11:2720–2730 ejn687
Rascol O et al (1997) The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain 120(Pt 1):103–110
Ruggiero DA, Anwar M, Golanov EV, Reis DJ (1997) The pedunculopontine tegmental nucleus issues collaterals to the fastigial nucleus and rostral ventrolateral reticular nucleus in the rat. Brain Res 760:272–276
Rye DB, Saper CB, Lee HJ, Wainer BH (1987) Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol 259:483–528. doi:10.1002/cne.902590403
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST (2000) CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, Parkinsonism and spinal cord injury. Neuropharmacology 39:777–787. pii: S0028390800000058
Schell GR, Strick PL (1984) The origin of thalamic inputs to the arcuate premotor and supplementary motor areas. J Neurosci 4:539–560
Schlerf JE, Verstynen TD, Ivry RB, Spencer RM (2010) Evidence of a novel somatopic map in the human neocerebellum during complex actions. J Neurophysiol 103:3330–3336. doi:10.1152/jn.01117.2009
Schmahmann JD, Pandya DN (1991) Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol 308:224–248. doi:10.1002/cne.903080209
Schweder PM, Hansen PC, Green AL, Quaghebeur G, Stein J, Aziz TZ (2010) Connectivity of the pedunculopontine nucleus in parkinsonian freezing of gait. NeuroReport 21:914–916. doi:10.1097/WNR.0b013e32833ce5f1
Sen S, Kawaguchi A, Truong Y, Lewis MM, Huang X (2010) Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson’s disease. Neuroscience 166:712–719. doi:10.1016/j.neuroscience.2009.12.036
Sestini S, Ramat S, Formiconi AR, Ammannati F, Sorbi S, Pupi A (2005) Brain networks underlying the clinical effects of long-term subthalamic stimulation for Parkinson’s disease: a 4-year follow-up study with rCBF SPECT. J Nucl Med 46:1444–1454 46/9/1444
Shi LH, Woodward DJ, Luo F, Anstrom K, Schallert T, Chang JY (2004) High-frequency stimulation of the subthalamic nucleus reverses limb-use asymmetry in rats with unilateral 6-hydroxydopamine lesions. Brain Res 1013:98–106. doi:10.1016/j.brainres.2004.03.053S0006899304005669
Shinoda Y, Kano M, Futami T (1985) Synaptic organization of the cerebello-thalamo-cerebral pathway in the cat. I. Projection of individual cerebellar nuclei to single pyramidal tract neurons in areas 4 and 6. Neurosci Res 2:133–156. pii:S0168-0102(85)90009-4
Sidtis JJ, Tagliati M, Alterman R, Sidtis D, Dhawan V, Eidelberg D (2012) Therapeutic high-frequency stimulation of the subthalamic nucleus in Parkinson’s disease produces global increases in cerebral blood flow. J Cereb Blood Flow Metab 32:41–49. doi:10.1038/jcbfm.2011.135
Sokolov AA, Erb M, Gharabaghi A, Grodd W, Tatagiba MS, Pavlova MA (2012) Biological motion processing: the left cerebellum communicates with the right superior temporal sulcus. NeuroImage 59:2824–2830. doi:10.1016/j.neuroimage.2011.08.039 S1053-8119(11)00938-4
Spann BM, Grofova I (1989) Origin of ascending and spinal pathways from the nucleus tegmenti pedunculopontinus in the rat. J Comp Neurol 283:13–27. doi:10.1002/cne.902830103
Stoodley CJ, Schmahmann JD (2009) Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage 44:489–501. doi:10.1016/j.neuroimage.2008.08.039 S1053-8119(08)00972-5
Stoodley CJ, Schmahmann JD (2010) Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex 46:831–844. doi:10.1016/j.cortex.2009.11.008 S0010-9452(09)00326-8
Strick PL, Dum RP, Fiez JA (2009) Cerebellum and nonmotor function. Annu Rev Neurosci 32:413–434. doi:10.1146/annurev.neuro.31.060407.125606
Sutton AC et al (2013a) Elevated potassium provides an ionic mechanism for deep brain stimulation in the hemiparkinsonian rat. Eur J Neurosci 37:231–241. doi:10.1111/ejn.12040
Sutton AC et al (2013b) Deep brain stimulation of the substantia nigra pars reticulata improves forelimb akinesia in the hemiparkinsonian rat. J Neurophysiol 109:363–374. doi:10.1152/jn.00311.2012
Takakusaki K, Shiroyama T, Kitai ST (1997) Two types of cholinergic neurons in the rat tegmental pedunculopontine nucleus: electrophysiological and morphological characterization. Neuroscience 79:1089–1109. pii: S0306-4522(97)00019-5
Thach WT, Goodkin HP, Keating JG (1992) The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci 15:403–442. doi:10.1146/annurev.ne.15.030192.002155
Thobois S, Ballanger B, Baraduc P, Le Bars D, Lavenne F, Broussolle E, Desmurget M (2007) Functional anatomy of motor urgency. NeuroImage 37:243–252. doi:10.1016/j.neuroimage.2007.04.049
Tsutsumi T, Houtani T, Toida K, Kase M, Yamashita T, Ishimura K, Sugimoto T (2007) Vesicular acetylcholine transporter-immunoreactive axon terminals enriched in the pontine nuclei of the mouse. Neuroscience 146:1869–1878. doi:10.1016/j.neuroscience.2007.03.019
Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST (2003) Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol 90:3958–3966. doi:10.1152/jn.00323.200300323.2003
Tykocki T, Mandat T, Nauman P (2011) Pedunculopontine nucleus deep brain stimulation in Parkinson’s disease. Arch Med Sci 7:555–564. doi:10.5114/aoms.2011.24119
Vajnerova O, Zhuravin IA, Brozek G (2000) Functional ablation of deep cerebellar nuclei temporarily impairs learned coordination of forepaw and tongue movements. Behav Brain Res 108:189–195. pii: S0166432899001473
van Donkelaar P, Stein JF, Passingham RE, Miall RC (2000) Temporary inactivation in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol 83:2780–2790
Wang HL, Morales M (2009) Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci 29:340–358. doi:10.1111/j.1460-9568.2008.06576.x
Wang J, Ma Y, Huang Z, Sun B, Guan Y, Zuo C (2010) Modulation of metabolic brain function by bilateral subthalamic nucleus stimulation in the treatment of Parkinson’s disease. J Neurol 257:72–78. doi:10.1007/s00415-009-5267-3
Weaver FM et al (2009) Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 301:63–73. doi:10.1001/jama.2008.929 301/1/63
Winn P (2006) How best to consider the structure and function of the pedunculopontine tegmental nucleus: evidence from animal studies. J Neurol Sci 248:234–250. doi:10.1016/j.jns.2006.05.036
Wu T, Hallett M (2013) The cerebellum in Parkinson’s disease. Brain 136:696–709. doi:10.1093/brain/aws360
Yu H, Sternad D, Corcos DM, Vaillancourt DE (2007) Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. NeuroImage 35:222–233. doi:10.1016/j.neuroimage.2006.11.047
Zhang QJ et al (2008) The firing activity of presumed cholinergic and non-cholinergic neurons of the pedunculopontine nucleus in 6-hydroxydopamine-lesioned rats: an in vivo electrophysiological study. Brain Res 1243:152–160. doi:10.1016/j.brainres.2008.09.028
Zhang J, Wang ZI, Baker KB, Vitek JL (2012) Effect of globus pallidus internus stimulation on neuronal activity in the pedunculopontine tegmental nucleus in the primate model of Parkinson’s disease. Exp Neurol 233:575–580. doi:10.1016/j.expneurol.2011.07.007
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sutton, A.C., O’Connor, K.A., Pilitsis, J.G. et al. Stimulation of the subthalamic nucleus engages the cerebellum for motor function in parkinsonian rats. Brain Struct Funct 220, 3595–3609 (2015). https://doi.org/10.1007/s00429-014-0876-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0876-8