Abstract
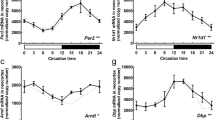
It is well-documented that circadian rhythms are controlled by the circadian master clock of the mammalian brain, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN clockwork is a cell autonomous mechanism consisting of a series of interlocked transcriptional/post-translational feedback loops. In turn, the SCN controls the seasonal rhythmicity of various biological processes, in particular the secretion pattern of hormones. Although the effects of gonadal hormones on circadian rhythmicity are clearly established, how the SCN integrates and regulates these hormonal stimuli remains unknown. We have previously found that clock genes are expressed in the choroid plexus (CP). Therefore, we compared the circadian expression of these genes in female and male rat CP. We show that there is a 24-h rhythm in the expression of Per2 and Cry2 in males and females. Bmal1 and Per1 expression also varied along the day, but only in females. Bmal1, Clock and Per1 mRNA did not show any significant differences in the CP of males. Moreover, data from cultured CP cells collected at different timepoints revealed significant circadian rhythms in mRNA abundance of Bmal1, Clock and Per2. In conclusion, our data show that the rat CP expresses all canonical clock genes and that their circadian expression differs between genders suggesting that hormones can regulate circadian rhythmicity in CP.






Similar content being viewed by others
References
Abe H, Honma S, Namihira M, Tanahashi Y, Ikeda M, Honma K (1998) Circadian rhythm and light responsiveness of BMAL1 expression, a partner of mammalian clock gene Clock, in the suprachiasmatic nucleus of rats. Neurosci Lett 258:93–96
Abe M, Herzog ED, Yamazaki S, Straume M, Tei H, Sakaki Y, Menaker M, Block GD (2002) Circadian rhythms in isolated brain regions. J Neurosci 22:350–356
Albrecht U (2012) Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74:246–260
Alvarez-Lopez C, Cernuda-Cernuda R, Garcia-Fernandez JM (2006) The mPer1 clock gene expression in the rd mouse suprachiasmatic nucleus is affected by the retinal degeneration. Brain Res 1087:134–141
Borgs L, Beukelaers P, Vandenbosch R, Nguyen L, Moonen G, Maquet P, Albrecht U, Belachew S, Malgrange B (2009) Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci 10:30
Chilov D, Hofer T, Bauer C, Wenger RH, Gassmann M (2001) Hypoxia affects expression of circadian genes PER1 and CLOCK in mouse brain. FASEB J 15:2613–2622
Chodobski A, Szmydynger-Chodobska J (2001) Choroid plexus: target for polypeptides and site of their synthesis. Microsc Res Tech 52:65–82
Daan S, Damassa D, Pittendrigh CS, Smith ER (1975) An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus). Proc Natl Acad Sci USA 72:3744–3747
De La Iglesia HO, Blaustein JD, Bittman EL (1999) Oestrogen receptor-alpha-immunoreactive neurones project to the suprachiasmatic nucleus of the female Syrian hamster. J Neuroendocrinol 11:481–490
Emerich DF, Skinner SJ, Borlongan CV, Vasconcellos AV, Thanos CG (2005) The choroid plexus in the rise, fall and repair of the brain. BioEssays 27:262–274
Fahrenkrug J, Hannibal J, Georg B (2008) Diurnal rhythmicity of the canonical clock genes Per1, Per2 and Bmal1 in the rat adrenal gland is unaltered after hypophysectomy. J Neuroendocrinol 20:323–329
Falcao AM, Marques F, Novais A, Sousa N, Palha JA, Sousa JC (2012) The path from the choroid plexus to the subventricular zone: go with the flow! Front Cell Neurosci 6:34
Guilding C, Piggins HD (2007) Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci 25:3195–3216
Guilding C, Hughes AT, Brown TM, Namvar S, Piggins HD (2009) A riot of rhythms: neuronal and glial circadian oscillators in the mediobasal hypothalamus. Mol Brain 2:28
Hastings MH, Field MD, Maywood ES, Weaver, Reppert SM (1999) Differential regulation of mPER1 and mTIM proteins in the mouse suprachiasmatic nuclei: new insights into a core clock mechanism. J Neurosci 19:RC11
Hastings MH, Reddy AB, Maywood ES (2003) A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 4:649–661
Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G (2011) The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol 39:186–212
Karatsoreos IN, Silver R (2007) Minireview: the neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology 148:5640–5647
Karman BN, Tischkau SA (2006) Circadian clock gene expression in the ovary: effects of luteinizing hormone. Biol Reprod 75:624–632
Ko CH, Takahashi JS (2006) Molecular components of the mammalian circadian clock. Hum Mol Genet 15(2):R271–R277
Kondratov RV, Chernov MV, Kondratova AA, Gorbacheva VY, Gudkov AV, Antoch MP (2003) BMAL1-dependent circadian oscillation of nuclear CLOCK: posttranslational events induced by dimerization of transcriptional activators of the mammalian clock system. Genes Dev 17:1921–1932
Kriegsfeld LJ, Silver R (2006) The regulation of neuroendocrine function: timing is everything. Horm Behav 49:557–574
Kwon I, Lee J, Chang SH, Jung NC, Lee BJ, Son GH, Kim K, Lee KH (2006) BMAL1 shuttling controls transactivation and degradation of the CLOCK/BMAL1 heterodimer. Mol Cell Biol 26:7318–7330
Li Y, Chen J, Chopp M (2002) Cell proliferation and differentiation from ependymal, subependymal and choroid plexus cells in response to stroke in rats. J Neurol Sci 193:137–146
Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445–462
Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R (2011) Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci 31:16107–16116
Moore RY (1983) Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc 42:2783–2789
Morin LP (1980) Effect of ovarian hormones on synchrony of hamster circadian rhythms. Physiol Behav 24:741–749
Murphy ZC, Pezuk P, Menaker M, Sellix MT (2013) Effects of ovarian hormones on internal circadian organization in rats. Biol Reprod 89:35
Nakamura TJ, Sellix MT, Menaker M, Block GD (2008) Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab 295:E1025–E1031
Nielsen HS, Hannibal J, Knudsen SM, Fahrenkrug J (2001) Pituitary adenylate cyclase-activating polypeptide induces period1 and period2 gene expression in the rat suprachiasmatic nucleus during late night. Neuroscience 103:433–441
Okamura H, Miyake S, Sumi Y, Yamaguchi S, Yasui A, Muijtjens M, Hoeijmakers JH, van der Horst GT (1999) Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science 286:2531–2534
Panda S, Hogenesch JB (2004) It’s all in the timing: many clocks, many outputs. J Biol Rhythms 19:374–387
Perrin JS, Segall LA, Harbour VL, Woodside B, Amir S (2006) The expression of the clock protein PER2 in the limbic forebrain is modulated by the estrous cycle. Proc Natl Acad Sci USA 103:5591–5596
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Quintela T, Alves CH, Goncalves I, Baltazar G, Saraiva MJ, Santos CR (2008) 5Alpha-dihydrotestosterone up-regulates transthyretin levels in mice and rat choroid plexus via an androgen receptor independent pathway. Brain Res 1229:18–26
Quintela T, Goncalves I, Carreto LC, Santos MA, Marcelino H, Patriarca FM, Santos CR (2013) Analysis of the effects of sex hormone background on the rat choroid plexus transcriptome by cDNA microarrays. PLoS ONE 8:e60199
Rath MF, Rohde K, Moller M (2012) Circadian oscillations of molecular clock components in the cerebellar cortex of the rat. Chronobiol Int 29:1289–1299
Rath MF, Rohde K, Fahrenkrug J, Moller M (2013) Circadian clock components in the rat neocortex: daily dynamics, localization and regulation. Brain Struct Funct 218:551–562
Reppert SM, Weaver DR (2001) Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol 63:647–676
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941
Sellix MT, Murphy ZC, Menaker M (2013) Excess androgen during puberty disrupts circadian organization in female rats. Endocrinology 154:1636–1647
Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, van der Horst GT, Hastings MH, Reppert SM (2000) Interacting molecular loops in the mammalian circadian clock. Science 288:1013–1019
Tamaru T, Isojima Y, van der Horst GT, Takei K, Nagai K, Takamatsu K (2003) Nucleocytoplasmic shuttling and phosphorylation of BMAL1 are regulated by circadian clock in cultured fibroblasts. Genes Cells 8:973–983
Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I (2008) Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. J Neuroendocrinol 20:1270–1277
Wunderer F, Kuhne S, Jilg A, Ackermann K, Sebesteny T, Maronde E, Stehle JH (2013) Clock gene expression in the human pituitary gland. Endocrinology 154(6):2046–2057
Yagita K, Tamanini F, Yasuda M, Hoeijmakers JH, van der Horst GT, Okamura H (2002) Nucleocytoplasmic shuttling and mCRY-dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBO J 21:1301–1314
Yamamoto T, Nakahata Y, Soma H, Akashi M, Mamine T, Takumi T (2004) Transcriptional oscillation of canonical clock genes in mouse peripheral tissues. BMC Mol Biol 5:18
Yamaoka S (1980) Modification of circadian sleep rhythms by gonadal steroids and the neural mechanisms involved. Brain Res 185:385–398
Zhang L, Abraham D, Lin ST, Oster H, Eichele G, Fu YH, Ptacek LJ (2012) PKCgamma participates in food entrainment by regulating BMAL1. Proc Natl Acad Sci USA 109:20679–20684
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (FCT, Portugal—http://www.FCT.pt) project grants (PTDC/SAU-NEU/114800/2009) and COMPETE (PEst-C/SAU/UI0709/2011). Telma Quintela is a recipient of a FCT fellowship (SFRH/BPD/70781/2010).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Quintela, T., Sousa, C., Patriarca, F.M. et al. Gender associated circadian oscillations of the clock genes in rat choroid plexus. Brain Struct Funct 220, 1251–1262 (2015). https://doi.org/10.1007/s00429-014-0720-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0720-1