Abstract
Low-frequency stimulation (LFS) is emerging as a new option for the treatment of epilepsy. The present study was designed to determine whether there is a crucial period for the treatment of epileptogenesis with LFS. LFS was delivered at different time-points to evaluate its anti-epileptogenic effect on amygdala-kindling rats. 18F-fluorodeoxyglucose small-animal positron-emission tomography (microPET) and multi-channel EEG recording (MER) were used to investigate the dynamics of brain networks during epileptogenesis and LFS treatment. Interestingly, LFS delivered in the first 7 days significantly retarded the progression of behavioral seizure stages and shortened the afterdischarge duration (ADD), LFS delivered throughout the whole process resulted in similar effects. However, if LFS was delivered at the beginning of seizure stage 2 or 3 (5 ± 0.3 days during kindling acquisition), it had no anti-epileptogenic effect and even prolonged the ADD and enhanced synchronization of the EEGs. MicroPET study revealed a notable hypometabolism in the amygdala, piriform cortex, entorhinal cortex and other regions in the limbic system during the period from seizure stage 0 to stage 2 or 3. The glucose metabolism in those regions was specifically increased by LFS. MER further verified that an early network of afterdischarge spread was formed in those brain regions during kindling acquisition. Thus, we provided direct evidence that modulation of the early network in the limbic system is crucial for the anti-epileptogenic effect of LFS in amygdaloid-kindling rats.







Similar content being viewed by others
References
Acharya MM, Hattiangady B, Shetty AK (2008) Progress in neuroprotective strategies for preventing epilepsy. Prog Neurobiol 84(4):363–404. doi:10.1016/j.pneurobio.2007.10.010
Armstrong C, Morgan RJ, Soltesz I (2009) Pursuing paradoxical proconvulsant prophylaxis for epileptogenesis. Epilepsia 50(7):1657–1669. doi:10.1111/j.1528-1167.2009.02173.x
Avoli M, D’Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V (2002) Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol 68(3):167–207
Berenyi A, Belluscio M, Mao D, Buzsaki G (2012) Closed-loop control of epilepsy by transcranial electrical stimulation. Science 337(6095):735–737. doi:10.1126/science.1223154
Bialer M, White HS (2010) Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov 9(1):68–82. doi:10.1038/nrd2997
Blumenfeld H (2003) From molecules to networks: cortical/subcortical interactions in the pathophysiology of idiopathic generalized epilepsy. Epilepsia 44(Suppl 2):7–15
Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR (2008) Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia 49(3):400–409. doi:10.1111/j.1528-1167.2007.01458.x
Burchfiel JL, Applegate CD (1989) Stepwise progression of kindling: perspectives from the kindling antagonism model. Neurosci Biobehav Rev 13(4):289–299
Carrington CA, Gilby KL, McIntyre DC (2007) Effect of focal low-frequency stimulation on amygdala-kindled afterdischarge thresholds and seizure profiles in fast- and slow-kindling rat strains. Epilepsia 48(8):1604–1613. doi:10.1111/j.1528-1167.2007.01077.x
Chassoux F, Semah F, Bouilleret V, Landre E, Devaux B, Turak B, Nataf F, Roux FX (2004) Metabolic changes and electro-clinical patterns in mesio-temporal lobe epilepsy: a correlative study. Brain 127(Pt 1):164–174. doi:10.1093/brain/awh014
D’Arcangelo G, Panuccio G, Tancredi V, Avoli M (2005) Repetitive low-frequency stimulation reduces epileptiform synchronization in limbic neuronal networks. Neurobiol Dis 19(1–2):119–128. doi:10.1016/j.nbd.2004.11.012
Dube C, Boyet S, Marescaux C, Nehlig A (2000) Progressive metabolic changes underlying the chronic reorganization of brain circuits during the silent phase of the lithium-pilocarpine model of epilepsy in the immature and adult Rat. Exp Neurol 162(1):146–157. doi:10.1006/exnr.2000.7324
Engel J Jr, Wolfson L, Brown L (1978) Anatomical correlates of electrical and behavioral events related to amygdaloid kindling. Ann Neurol 3(6):538–544. doi:10.1002/ana.410030615
Faingold CL (2004) Emergent properties of CNS neuronal networks as targets for pharmacology: application to anticonvulsant drug action. Prog Neurobiol 72(1):55–85. doi:10.1016/j.pneurobio.2003.11.003
Feddersen B, Vercueil L, Noachtar S, David O, Depaulis A, Deransart C (2007) Controlling seizures is not controlling epilepsy: a parametric study of deep brain stimulation for epilepsy. Neurobiol Dis 27(3):292–300. doi:10.1016/j.nbd.2007.05.005
Gao F, Guo Y, Zhang H, Wang S, Wang J, Wu JM, Chen Z, Ding MP (2009) Anterior thalamic nucleus stimulation modulates regional cerebral metabolism: an FDG-MicroPET study in rats. Neurobiol Dis 34(3):477–483. doi:10.1016/j.nbd.2009.03.001
Geyer JD, Bilir E, Faught RE, Kuzniecky R, Gilliam F (1999) Significance of interictal temporal lobe delta activity for localization of the primary epileptogenic region. Neurology 52(1):202–205
Gjedde A, Marrett S, Vafaee M (2002) Oxidative and nonoxidative metabolism of excited neurons and astrocytes. J Cereb Blood Flow Metab 22(1):1–14. doi:10.1097/00004647-200201000-00001
Graber KD, Fisher RS (2012) Deep brain stimulation for epilepsy: animal models. In: Noebels JL, Avoli M, Rogawski MA, et al (eds) Jasper’s basic mechanisms of the epilepsies [internet], 4th edn. National Center for Biotechnology Information (US), Bethesda (MD). Available from: http://www.ncbi.nlm.nih.gov/books/NBK98160/
Graber KD, Prince DA (2004) A critical period for prevention of posttraumatic neocortical hyperexcitability in rats. Ann Neurol 55(6):860–870. doi:10.1002/ana.20124
Grill WM, Snyder AN, Miocinovic S (2004) Deep brain stimulation creates an informational lesion of the stimulated nucleus. NeuroReport 15(7):1137–1140
Gubellini P, Salin P, Kerkerian-Le Goff L, Baunez C (2009) Deep brain stimulation in neurological diseases and experimental models: from molecule to complex behavior. Prog Neurobiol 89(1):79–123. doi:10.1016/j.pneurobio.2009.06.003
Guo Y, Gao F, Wang S, Ding Y, Zhang H, Wang J, Ding MP (2009) In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience 162(4):972–979. doi:10.1016/j.neuroscience.2009.05.041
Hewapathirane DS, Burnham WM (2005) Propagation of amygdala-kindled seizures to the hippocampus in the rat: electroencephalographic features and behavioural correlates. Neurosci Res 53(4):369–375. doi:10.1016/j.neures.2005.08.007
Jiruska P, Powell AD, Deans JK, Jefferys JG (2010) Effects of direct brain stimulation depend on seizure dynamics. Epilepsia 51(Suppl 3):93–97. doi:10.1111/j.1528-1167.2010.02619.x
Jueptner M, Weiller C (1995) Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage 2(2):148–156
Jupp B, Williams J, Binns D, Hicks RJ, Cardamone L, Jones N, Rees S, O’Brien TJ (2012) Hypometabolism precedes limbic atrophy and spontaneous recurrent seizures in a rat model of TLE. Epilepsia 53(7):1233–1244. doi:10.1111/j.1528-1167.2012.03525.x
Kahane P, Depaulis A (2010) Deep brain stimulation in epilepsy: what is next? Curr Opin Neurol 23(2):177–182. doi:10.1097/WCO.0b013e3283374a39
Knowlton RC, Laxer KD, Klein G, Sawrie S, Ende G, Hawkins RA, Aassar OS, Soohoo K, Wong S, Barbaro N (2001) In vivo hippocampal glucose metabolism in mesial temporal lobe epilepsy. Neurology 57(7):1184–1190
Kornblum HI, Araujo DM, Annala AJ, Tatsukawa KJ, Phelps ME, Cherry SR (2000) In vivo imaging of neuronal activation and plasticity in the rat brain by high resolution positron emission tomography (microPET). Nat Biotechnol 18(6):655–660. doi:10.1038/76509
Kramer MA, Cash SS (2012) Epilepsy as a disorder of cortical network organization. Neuroscientist 18(4):360–372. doi:10.1177/1073858411422754
Langer M, Brandt C, Zellinger C, Loscher W (2011) Therapeutic window of opportunity for the neuroprotective effect of valproate versus the competitive AMPA receptor antagonist NS1209 following status epilepticus in rats. Neuropharmacology 61(5–6):1033–1047. doi:10.1016/j.neuropharm.2011.06.015
Le Gal La Salle, G (1981) Amygdaloid kindling in the rat: regional differences and general properties. In: Wada JA (ed) Kindling 2. Raven Press, New York, pp 31–47
Lee EM, Park GY, Im KC, Kim ST, Woo CW, Chung JH, Kim KS, Kim JS, Shon YM, Kim YI, Kang JK (2012) Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia 53(5):860–869. doi:10.1111/j.1528-1167.2012.03432.x
Lockman J, Fisher RS (2009) Therapeutic brain stimulation for epilepsy. Neurol Clin 27(4):1031–1040. doi:10.1016/j.ncl.2009.06.005
Loscher W, Schmidt D (2006) New Horizons in the development of antiepileptic drugs: innovative strategies. Epilepsy Res 69(3):183–272
Mani R, Pollard J, Dichter MA (2011) Human clinical trails in antiepileptogenesis. Neurosci Lett 497(3):251–256. doi:10.1016/j.neulet.2011.03.010
McIntyre CC, Hahn PJ (2010) Network perspectives on the mechanisms of deep brain stimulation. Neurobiol Dis 38(3):329–337. doi:10.1016/j.nbd.2009.09.022
McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL (2004) Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol 115 (6):1239–1248. doi:10.1016/j.clinph.2003.12.024
Molnar GF, Sailer A, Gunraj CA, Cunic DI, Wennberg RA, Lozano AM, Chen R (2006) Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology 66(4):566–571. doi:10.1212/01.wnl.0000198254.08581.6b
Morimoto K, Fahnestock M, Racine RJ (2004) Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol 73(1):1–60. doi:10.1016/j.pneurobio.2004.03.009
Moss J, Ryder T, Aziz TZ, Graeber MB, Bain PG (2004) Electron microscopy of tissue adherent to explanted electrodes in dystonia and Parkinson’s disease. Brain 127(Pt 12):2755–2763. doi:10.1093/brain/awh292
Nehlig A, Coles JA (2007) Cellular pathways of energy metabolism in the brain: is glucose used by neurons or astrocytes? Glia 55(12):1238–1250. doi:10.1002/glia.20376
Otsuki K, Morimoto K, Sato K, Yamada N, Kuroda S (1998) Effects of lamotrigine and conventional antiepileptic drugs on amygdala- and hippocampal-kindled seizures in rats. Epilepsy Res 31(2):101–112
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Academic Press/Elsevier, Amsterdam, Boston
Pitkanen A, Narkilahti S, Bezvenyuk Z, Haapalinna A, Nissinen J (2004) Atipamezole, an alpha(2)-adrenoceptor antagonist, has disease modifying effects on epileptogenesis in rats. Epilepsy Res 61(1–3):119–140. doi:10.1016/j.eplepsyres.2004.07.005
Racine RJ (1972) Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol 32(3):281–294
Reiher J, Beaudry M, Leduc CP (1989) Temporal intermittent rhythmic delta activity (TIRDA) in the diagnosis of complex partial epilepsy: sensitivity, specificity and predictive value. Can J Neurol Sci 16(4):398–401
Rocher AB, Chapon F, Blaizot X, Baron JC, Chavoix C (2003) Resting-state brain glucose utilization as measured by PET is directly related to regional synaptophysin levels: a study in baboons. Neuroimage 20(3):1894–1898
Sato M, Racine RJ, McIntyre DC (1990) Kindling: basic mechanisms and clinical validity. Electroencephalogr Clin Neurophysiol 76(5):459–472
Schindler K, Elger CE, Lehnertz K (2007) Changes of EEG synchronization during low-frequency electric stimulation of the seizure onset zone. Epilepsy Res 77(2–3):108–119. doi:10.1016/j.eplepsyres.2007.09.011
Skarpaas TL, Morrell MJ (2009) Intracranial stimulation therapy for epilepsy. Neurotherapeutics 6(2):238–243. doi:10.1016/j.nurt.2009.01.022
Sun HL, Zhang SH, Zhong K, Xu ZH, Zhu W, Fang Q, Wu DC, Hu WW, Xiao B, Chen Z (2010) Mode-dependent effect of low-frequency stimulation targeting the hippocampal CA3 subfield on amygdala-kindled seizures in rats. Epilepsy Res 90(1–2):83–90. doi:10.1016/j.eplepsyres.2010.03.011
Takebayashi S, Hashizume K, Tanaka T, Hodozuka A (2007) The effect of electrical stimulation and lesioning of the anterior thalamic nucleus on kainic acid-induced focal cortical seizure status in rats. Epilepsia 48(2):348–358. doi:10.1111/j.1528-1167.2006.00948.x
Temkin NR (2001) Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia 42(4):515–524
Ullal GR, Ninchoji T, Uemura K (1989) Low frequency stimulation induces an increase in after-discharge thresholds in hippocampal and amygdaloid kindling. Epilepsy Res 3(3):232–235
Velisek L, Veliskova J, Stanton PK (2002) Low-frequency stimulation of the kindling focus delays basolateral amygdala kindling in immature rats. Neurosci Lett 326(1):61–63
Vonck K, Boon P, Achten E, De Reuck J, Caemaert J (2002) Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol 52(5):556–565. doi:10.1002/ana.10323
Wang S, Wu DC, Fan XN, Zhu MZ, Hu QY, Zhou D, Ding MP, Chen Z (2010) Mediodorsal thalamic stimulation is not protective against seizures induced by amygdaloid kindling in rats. Neurosci Lett 481(2):97–101. doi:10.1016/j.neulet.2010.06.060
Weiss SR, Eidsath A, Li XL, Heynen T, Post RM (1998) Quenching revisited: low level direct current inhibits amygdala-kindled seizures. Exp Neurol 154(1):185–192. doi:10.1006/exnr.1998.6932
Wu DC, Xu ZH, Wang S, Fang Q, Hu DQ, Li Q, Sun HL, Zhang SH, Chen Z (2008) Time-dependent effect of low-frequency stimulation on amygdaloid-kindling seizures in rats. Neurobiol Dis 31(1):74–79. doi:10.1016/j.nbd.2008.03.007
Xu ZH, Wu DC, Fang Q, Zhong K, Wang S, Sun HL, Zhang SH, Chen Z (2010) Therapeutic time window of low-frequency stimulation at entorhinal cortex for amygdaloid-kindling seizures in rats. Epilepsia 51(9):1861–1864. doi:10.1111/j.1528-1167.2010.02663.x
Xu Z, Wang Y, Jin M, Yue J, Xu C, Ying X, Wu D, Zhang S, Chen Z (2012) Polarity-dependent effect of low-frequency stimulation on amygdaloid kindling in rats. Brain Stimul. doi:10.1016/j.brs.2012.04.010
Yang LX, Jin CL, Zhu-Ge ZB, Wang S, Wei EQ, Bruce IC, Chen Z (2006) Unilateral low-frequency stimulation of central piriform cortex delays seizure development induced by amygdaloid kindling in rats. Neuroscience 138(4):1089–1096. doi:10.1016/j.neuroscience.2005.12.006
Zhong K, Wu DC, Jin MM, Xu ZH, Wang Y, Hou WW, Li XM, Zhang SH, Chen Z (2012) Wide therapeutic time-window of low-frequency stimulation at the subiculum for temporal lobe epilepsy treatment in rats. Neurobiol Dis 48(1):20–26. doi:10.1016/j.nbd.2012.05.011
Acknowledgments
This work was funded by the National Natural Science Foundation of China (81030061, 81221003, 81273492, 81171227 and 81000556) and partly by the Program for Changjiang Scholars and Innovative Research Team in University. We are grateful to Dr. IC Bruce for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yi Wang and Zhenghao Xu have contributed equally to the paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
429_2013_594_MOESM1_ESM.tif
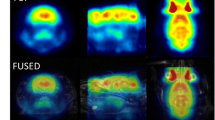
Supplementary Fig. 1 Co-registration of microPET and MRI images. 6 of 17 slices of coronal views of the rat brains are displayed. The regions of interest (ROIs) are drawn according to the anatomical structures and are numbered. The ROIs correspond to the following brain regions: 1 prefrontal cortex, 2 sensorimotor cortex, 3 striatum, 4 piriform cortex, 5 hippocampus, 6 amygdala, 7 thalamus, 8 visual cortex, 9 auditory cortex, 10 entorhinal cortex, 11 pons, 12 cerebellum (TIFF 6307 kb)
429_2013_594_MOESM2_ESM.tif
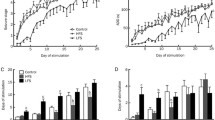
Supplementary Fig. 2 Effects on kindling acquisition of focal LFS delivered in whole kindling process. a–j Effects of LFS on the behavioral stage of seizures during kindling acquisition for each rat (n = 10). k Number of stimulations required to reach each seizure stage and (1) required to reach stage 5 from seizure stage 2 or 3 during kindling acquisition. Only the rat reaching generalized seizures were analyzed. **P < 0.01 represents differences compared with Control group. The t test was used for statistical analysis of k and j (TIFF 1332 kb)
429_2013_594_MOESM3_ESM.tif
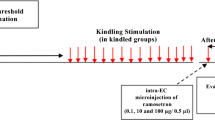
Supplementary Fig. 3 Effects of LFS (black bar, n = 6) on fully kindled rats compared to Control rats (white bar, n = 7). The mean incidence (a), latency (b), cumulative generalized seizures duration (c) and cumulative ADD (d) during 7 days of LFS treatment. **P < 0.01 compared with Control group. The t test was used for b, c and d. The χ 2 test was used for comparison of generalized seizure incidence (TIFF 488 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Xu, Z., Cheng, H. et al. Low-frequency stimulation inhibits epileptogenesis by modulating the early network of the limbic system as evaluated in amygdala kindling model. Brain Struct Funct 219, 1685–1696 (2014). https://doi.org/10.1007/s00429-013-0594-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-013-0594-7