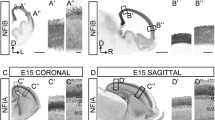
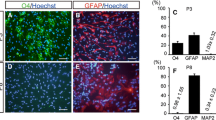
Abstract
The transcription factor Pax6 has been reported to specify neural progenitor cell fates during development and maintain neuronal commitments in the adult. The spatiotemporal patterns of Pax6 expression were examined in sagittal and horizontal sections of the embryonic, postnatal, and adult brains using immunohistochemistry and double immunolabeling. The proportion of Pax6-immunopositive cells in various parts of the adult brain was estimated using the isotropic fractionator methodology. It was shown that at embryonic day 11 (E11) Pax6 was robustly expressed in the proliferative neuroepithelia of the ventricular zone in the forebrain and hindbrain, and in the floor and the mesencephalic reticular formation (mRt) in the midbrain. At E12, its expression emerged in the nucleus of the lateral lemniscus in the rhombencephalon and disappeared from the floor of the midbrain. As neurodevelopment proceeds, the expression pattern of Pax6 changes from the mitotic germinal zone in the ventricular zone to become extensively distributed in cell groups in the forebrain and hindbrain, and the expression persisted in the mRt. The majority of Pax6-positive cell groups were maintained until adult life, but the intensity of Pax6 expression became much weaker. Pax6 expression was maintained in the mitotic subventricular zone in the adult brain, but not in the germinal region dentate gyrus in the adult hippocampus. There was no obvious colocalization of Pax6 and NeuN during embryonic development, suggesting Pax6 is found primarily in developing progenitor cells. In the adult brain, however, Pax6 maintains neuronal features of some subtypes of neurons, as indicated by 97.1% of Pax6-positive cells co-expressing NeuN in the cerebellum, 40.7% in the olfactory bulb, 38.3% in the cerebrum, and 73.9% in the remaining brain except the hippocampus. Differentiated tyrosine hydroxylase (TH) neurons were observed in the floor of the E11 midbrain where Pax6 was also expressed, but no obvious colocaliztion of TH and Pax6 was detected. No Pax6 expression was observed in TH-expressing areas in the midbrain at E12, E14, and postnatal day 1. These results support the notion that Pax6 plays pivotal roles in specifying neural progenitor cell commitments and maintaining certain mature neuronal fates.










Similar content being viewed by others
Abbreviations
- 3V:
-
Third ventricle
- 4V:
-
Fourth ventricle
- 5PC:
-
Motor trigeminal nucleus, parvicellular part
- AA:
-
Anterior amygdaloid area
- Amg:
-
Amygdala
- AO:
-
Anterior olfactory nucleus
- AOP:
-
Anterior olfactory area posterior part
- APT:
-
Anterior pretectal nucleus
- BL:
-
Basolateral amygdaloid nucleus
- BM:
-
Basomedial amygdaloid nucleus
- Cb:
-
Cerebellum
- Ce:
-
Central amygdaloid nucleus
- Ceph:
-
Cephalic flexure
- CG:
-
Central gray
- Cx:
-
Cortex
- DC:
-
Dorsal cochlear nucleus
- DG:
-
Dentate gyrus
- Dien:
-
Diencephalon
- DLL:
-
Dorsal nucleus of the lateral lemniscus
- DR:
-
Dorsal raphe nucleus
- DTg:
-
Dorsal tegmental nucleus
- DTT:
-
Dorsal tenia tecta
- ECu:
-
External cuneate nucleus
- EGL:
-
External granular layer of developing cerebellum
- EP:
-
Entopeduncular nucleus
- E/OV:
-
Ependymal and subendymal layer/olfactory ventricle
- F:
-
Nucleus of the fields of Forel
- FovIs:
-
Fovea isthmus
- Gi:
-
Gigantocellular reticular nucleus
- Gl:
-
Glomerular layer of the olfactory bulb
- GP:
-
Globus pallidus
- GrC:
-
Granule cell layer of cochlear nuclei
- HDB:
-
Nucleus of the horizontal limb of the diagonal band
- I:
-
Intercalated nuclei of the amygdala
- IEn:
-
Intermediate endopiriform nucleus
- InC:
-
Interstitial nucleus of Cajal
- IO:
-
Inferior olivary nucleus
- Is:
-
Isthmus
- isRt:
-
isthmic reticular formation
- JPLH:
-
Juxtaparaventricular part of lateral hypothalamus
- La:
-
Lateral amygdaloid nucleus
- LC:
-
Locus coeruleus
- LD:
-
Laterodorsal thalamic nucleus
- LDTg:
-
Laterodorsal tegmental nucleus
- LHb:
-
Lateral habenular nucleus
- Li:
-
Linear nucleus
- LL:
-
Nucleus of the lateral lemniscus
- LP:
-
Lateral posterior thalamic nucleus
- LPO:
-
Lateral preoptic area
- LRt:
-
Lateral reticular nucleus
- LSI:
-
Lateral septal nucleus, intermediate part
- LV:
-
Lateral ventricle
- MCPC:
-
Magnocellular nucleus of the posterior commissure
- Me:
-
Medial amygdaloid nucleus
- Mesen:
-
Mesencephalon
- MHb:
-
Medial habenular nucleus
- MnR:
-
Median raphe nucleus
- mRt:
-
mesencephalic reticular formation
- MS:
-
Medial septal nucleus
- MVe:
-
Medial vestibular nucleus
- MVPO:
-
Medioventral periolivary nucleus
- PAG:
-
Periaqueductal gray
- PaXi:
-
Paraxiphoid nucleus of thalamus
- Pc:
-
Posterior commissure
- PCom:
-
Nucleus of the posterior commissure
- PDTg:
-
Posterodorsal tegmental nucleus
- Pir:
-
Piriform cortex
- PL:
-
Paralemniscal nucleus
- PLH:
-
Peduncular part of lateral hypothalamus
- Pn:
-
Pontine nuclei
- PrC:
-
Precommissural nucleus
- PrG:
-
Pregeniculate nucleus
- PrTh:
-
Prethalamus (prosomere 3)
- PrThE:
-
Prethalamic eminence
- Ptec:
-
Pretectum
- Rhomb:
-
Rhombencephalon
- Rt:
-
Reticular thalamic nucleus
- RtTg:
-
Reticulotegmental nucleus of the pons
- SN:
-
Substantia nigra
- SPFPC:
-
Subparafascicular thalamic nucleus, parvicellular part
- SubG:
-
Subgeniculate nucleus
- SuVe:
-
Superior vestibular nucleus
- Telen:
-
Telencephalon
- Tu:
-
Olfactory tubercle
- VC:
-
Ventral cochlear nucleus
- VCA:
-
Ventral cochlear nucleus, anterior part
- VDB:
-
Nucleus of the vertical limb of the diagonal band
- VEn:
-
Ventral endopiriform claustrum
- VP:
-
Ventral pallidum
- VTT:
-
Ventral tenia tecta
- VTA:
-
Ventral tegmental area
- VZ:
-
Ventricular zone
- X:
-
Nucleus X
- ZI:
-
Zona incerta
References
Ashwell KW, Paxinos G (2008) Atlas of the developing rat nervous system, 3rd edn. Elsevier Academic Press, San Diego
Backman M, Machon O, Mygland L, van den Bout CJ, Zhong W, Taketo MM, Krauss S (2005) Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev Biol 279(1):155–168. doi:10.1016/j.ydbio.2004.12.010
Burri M, Tromvoukis Y, Bopp D, Frigerio G, Noll M (1989) Conservation of the paired domain in metazoans and its structure in three isolated human genes. EMBO J 8(4):1183–1190
Callaerts P, Halder G, Gehring WJ (1997) PAX-6 in development and evolution. Annu Rev Neurosci 20:483–532. doi:10.1146/annurev.neuro.20.1.483
Franklin K, Paxinos G (2008) The mouse brain in stereotaxic coordinates, 3rd edn. Academic Press, San Diego
Fu Y, Tvrdik P, Makki N, Palombi O, Machold R, Paxinos G, Watson C (2009) The precerebellar linear nucleus in the mouse defined by connections, immunohistochemistry, and gene expression. Brain Res 1271:49–59. doi:10.1016/j.brainres.2009.02.068
Fu Y, Tvrdik P, Makki N, Paxinos G, Watson C (2011) Precerebellar cell groups in the hindbrain of the mouse defined by retrograde tracing and correlated with cumulative Wnt1-cre genetic labeling. Cerebellum 10(3):570–584. doi:10.1007/s12311-011-0266-1
Georgala PA, Carr CB, Price DJ (2011) The role of Pax6 in forebrain development. Dev Neurobiol 71(8):690–709. doi:10.1002/dneu.20895
Hauptmann G, Gerster T (2000) Regulatory gene expression patterns reveal transverse and longitudinal subdivisions of the embryonic zebrafish forebrain. Mech Dev 91(1–2):105–118
Herculano-Houzel S, Lent R (2005) Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci: Official J Soc Neurosci 25(10):2518–2521. doi:10.1523/jneurosci.4526-04.2005
Hufnagel RB, Riesenberg AN, Saul SM, Brown NL (2007) Conserved regulation of Math5 and Math1 revealed by Math5-GFP transgenes. Mol Cell Neurosci 36(4):435–448. doi:10.1016/j.mcn.2007.08.006
Kawakami A, Kimura-Kawakami M, Nomura T, Fujisawa H (1997) Distributions of PAX6 and PAX7 proteins suggest their involvement in both early and late phases of chick brain development. Mech Dev 66(1–2):119–130
Landsberg RL, Awatramani RB, Hunter NL, Farago AF, DiPietrantonio HJ, Rodriguez CI, Dymecki SM (2005) Hindbrain rhombic lip is comprised of discrete progenitor cell populations allocated by Pax6. Neuron 48(6):933–947. doi:10.1016/j.neuron.2005.11.031
Liang H, Paxinos G, Watson C (2010) Projections from the brain to the spinal cord in the mouse. Brain Struct Funct 215(3–4):159–186. doi:10.1007/s00429-010-0281-x
Liu A, Joyner AL (2001) Early anterior/posterior patterning of the midbrain and cerebellum. Annu Rev Neurosci 24:869–896. doi:10.1146/annurev.neuro.24.1.869
Machold R, Fishell G (2005) Math1 is expressed in temporally discrete pools of cerebellar rhombic-lip neural progenitors. Neuron 48(1):17–24. doi:10.1016/j.neuron.2005.08.028
Machon O, Kreslova J, Ruzickova J, Vacik T, Klimova L, Fujimura N, Lachova J, Kozmik Z (2010) Lens morphogenesis is dependent on Pax6-mediated inhibition of the canonical Wnt/beta-catenin signaling in the lens surface ectoderm. Genesis 48(2):86–95. doi:10.1002/dvg.20583
Manuel M, Price D (2005) Role of Pax6 in forebrain regionalization. Brain Res Bull 66(4–6):387–393. doi:10.1016/j.brainresbull.2005.02.006
Mastick GS, Davis NM, Andrew GL, Easter SS Jr (1997) Pax-6 functions in boundary formation and axon guidance in the embryonic mouse forebrain. Development 124(10):1985–1997
Metin C, Alvarez C, Moudoux D, Vitalis T, Pieau C, Molnar Z (2007) Conserved pattern of tangential neuronal migration during forebrain development. Development 134(15):2815–2827. doi:10.1242/dev.02869
Mo Z, Zecevic N (2008) Is Pax6 critical for neurogenesis in the human fetal brain? Cereb Cortex 18(6):1455–1465. doi:10.1093/cercor/bhm181
Moreno N, Rétaux S, González A (2008) Spatio-temporal expression of Pax6 in Xenopus forebrain. Brain Res 1239:92–99. doi:10.1016/j.brainres.2008.08.052
Muzio L, Mallamaci A (2003) Emx1, emx2 and pax6 in specification, regionalization and arealization of the cerebral cortex. Cereb Cortex 13(6):641–647
Nacher J, Varea E, Blasco-Ibanez JM, Castillo-Gomez E, Crespo C, Martinez-Guijarro FJ, McEwen BS (2005) Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J Neurosci Res 81(6):753–761. doi:10.1002/jnr.20596
Noll M (1993) Evolution and role of Pax genes. Curr Opin Genet Dev 3(4):595–605
Osorio J, Mazan S, Retaux S (2005) Organisation of the lamprey (Lampetra fluviatilis) embryonic brain: insights from LIM-homeodomain, Pax and hedgehog genes. Dev Biol 288(1):100–112. doi:10.1016/j.ydbio.2005.08.042
Osumi N (2001) The role of Pax6 in brain patterning. Tohoku J Exp Med 193(3):163–174
Osumi N, Shinohara H, Numayama-Tsuruta K, Maekawa M (2008) Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells 26(7):1663–1672. doi:10.1634/stemcells.2007-0884
Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates, 6th edn. Elsevier Academic Press, San Diego
Paxinos G, Halliday G, Watson C, Koutcherov Y, Wang H (2007) Atlas of the developing mouse brain at E17.5, P0, and P6. Elsevier Academic Press, San Diego
Pritz MB, Ruan YW (2009) PAX6 immunoreactivity in the diencephalon and midbrain of alligator during early development. Brain Behav Evol 73(1):1–15. doi:10.1159/000195695
Puelles L, Rubenstein JL (2003) Forebrain gene expression domains and the evolving prosomeric model. Trends Neurosci 26(9):469–476
Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL (2000) Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol 424(3):409–438
Puelles L, Martinez-de-la-Torre M, Paxinos G, Watson C, Martinez S (2007) The chick brain in stereotaxic coordinates: an atlas featuring neuromeres and mammalian homologies. Elsevier Academic Press, San Diego
Sansom SN, Griffiths DS, Faedo A, Kleinjan DJ, Ruan Y, Smith J, van Heyningen V, Rubenstein JL, Livesey FJ (2009) The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS genetics 5(6):e1000511. doi:10.1371/journal.pgen.1000511
Schubert FR, Lumsden A (2005) Transcriptional control of early tract formation in the embryonic chick midbrain. Development 132(8):1785–1793. doi:10.1242/dev.01731
Schuller U, Rowitch DH (2007) Beta-catenin function is required for cerebellar morphogenesis. Brain Res 1140:161–169. doi:10.1016/j.brainres.2006.05.105
Sillitoe R, Fu Y, Watson C (2012) Cerebellum. In: Watson C, Paxinos G, Puelles L (eds) The mouse nervous system. Elsevier Academic Press, San Diego, pp 360–396
Spitere K, Toulouse A, O’Sullivan DB, Sullivan AM (2008) TAT-PAX6 protein transduction in neural progenitor cells: a novel approach for generation of dopaminergic neurones in vitro. Brain Res 1208:25–34. doi:10.1016/j.brainres.2008.02.065
Stoykova A, Gruss P (1994) Roles of Pax-genes in developing and adult brain as suggested by expression patterns. J Neurosci 14(3 Pt 2):1395–1412
Swanson DJ, Tong Y, Goldowitz D (2005) Disruption of cerebellar granule cell development in the Pax6 mutant, Sey mouse. Brain Res Dev Brain Res 160(2):176–193. doi:10.1016/j.devbrainres.2005.09.005
Takahashi M, Osumi N (2011) Pax6 regulates boundary-cell specification in the rat hindbrain. Mech Dev. doi:10.1016/j.mod.2011.04.001
Terzic J, Saraga-Babic M (1999) Expression pattern of PAX3 and PAX6 genes during human embryogenesis. Internat J Dev Biol 43(6):501–508
Tole S, Remedios R, Saha B, Stoykova A (2005) Selective requirement of Pax6, but not Emx2, in the specification and development of several nuclei of the amygdaloid complex. J Neurosci 25(10):2753–2760. doi:10.1523/JNEUROSCI.3014-04.2005
Vitalis T, Cases O, Engelkamp D, Verney C, Price DJ (2000) Defect of tyrosine hydroxylase-immunoreactive neurons in the brains of mice lacking the transcription factor Pax6. J Neurosci 20(17):6501–6516. (pii:20/17/6501)
Walther C, Gruss P (1991) Pax-6, a murine paired box gene, is expressed in the developing CNS. Development 113(4):1435–1449
Watson C (2010) The presumptive isthmic region in a mouse as defined by fgf8 expression. Brain Behav Evol 75:15
Wexler EM, Paucer A, Kornblum HI, Palmer TD, Geschwind DH (2009) Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells 27(5):1130–1141. doi:10.1002/stem.36
Wrobel CN, Mutch CA, Swaminathan S, Taketo MM, Chenn A (2007) Persistent expression of stabilized beta-catenin delays maturation of radial glial cells into intermediate progenitors. Dev Biol 309(2):285–297. doi:10.1016/j.ydbio.2007.07.013
Wullimann MF, Rink E (2001) Detailed immunohistology of Pax6 protein and tyrosine hydroxylase in the early zebrafish brain suggests role of Pax6 gene in development of dopaminergic diencephalic neurons. Brain Res Dev Brain Res 131(1–2):173–191
Yamada K, Semba R, Ding X, Ma N, Nagahama M (2005) Discrimination of cell nuclei in early S-phase, mid-to-late S-phase, and G(2)/M-phase by sequential administration of 5-bromo-2′-deoxyuridine and 5-chloro-2′-deoxyuridine. J Histochem Cytochem 53(11):1365–1370. doi:10.1369/jhc.4A6601.2005
Zhang X, Huang CT, Chen J, Pankratz MT, Xi J, Li J, Zhang S-C (2010) Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7(1):90–100. doi:10.1016/j.stem.2010.04.017
Acknowledgments
We thank Dr. Huazheng Liang, Dr. Emma Schofield, and Mr Hua Zhao for technical support. This work was supported by the Christopher and Dana Reeve Foundation and an Australia Fellowship awarded to Professor George Paxinos by the National Health and Medical Research Council (NHMRC) (466028).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duan, D., Fu, Y., Paxinos, G. et al. Spatiotemporal expression patterns of Pax6 in the brain of embryonic, newborn, and adult mice. Brain Struct Funct 218, 353–372 (2013). https://doi.org/10.1007/s00429-012-0397-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-012-0397-2