Abstract
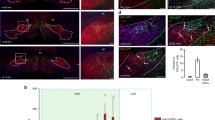
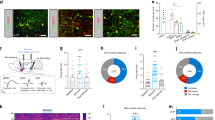
The nucleus accumbens (NAc) is positioned to integrate signals originating from limbic and cortical areas and to modulate reward-related motor output of various goal-directed behaviours. The major target of the NAc GABAergic output neurons is the ventral pallidum (VP). VP is part of the reward circuit and controls the ascending mesolimbic dopamine system, as well as the motor output structures and the brainstem. The excitatory inputs governing this system converge in the NAc from the prefrontal cortex (PFC), ventral hippocampus (vHC), midline and intralaminar thalamus (TH) and basolateral nucleus of the amygdala (BLA). It is unclear which if any of these afferents innervate the medium spiny neurons of the NAc, that project to the VP. To identify the source of glutamatergic afferents that innervate neurons projecting to the VP, a dual-labelling method was used: Phaseolus vulgaris leucoagglutinin for anterograde and EGFP-encoded adenovirus for retrograde tract-tracing. Within the NAc, anterogradely labelled BLA terminals formed asymmetric synapses on dendritic spines that belonged to medium spiny neurons retrogradely labelled from the VP. TH terminals also formed synapses on dendritic spines of NAc neurons projecting to the VP. However, dendrites and dendritic spines retrogradely labelled from VP received no direct synaptic contacts from afferents originating from mPFC and vHC in the present material, despite the large number of fibres labelled by the anterograde tracer injections. These findings represent the first experimental evidence for a selective glutamatergic innervation of NAc neurons projecting to the VP. The glutamatergic inputs of different origin (i.e. mPFC, vHC, BLA, TH) to the NAc might thus convey different types of reward-related information during goal-directed behaviour, and thereby contribute to the complex regulation of nucleus accumbens functions.





Similar content being viewed by others
Abbreviations
- NAc:
-
Nucleus accumbens
- mPFC:
-
Medial prefrontal cortex
- vHC:
-
Ventral hippocampus
- BLA:
-
Basolateral amygdala
- PVT:
-
Paraventricular thalamic nucleus
- VP:
-
Ventral pallidum
- VTA:
-
Ventral tegmental area
- PB:
-
Phosphate buffer
- TBS:
-
Tris-buffered saline
- PHAL :
-
Phaseolus vulgaris leucoagglutinin
- EGFP:
-
Enhanced green fluorescent protein AdSynEGFP adenovirus vector using a neuron-specific synapsin I Promoter and enhanced green fluorescent protein reporter (EGFP)
- vGluT2:
-
Vesicular glutamate transporter 2 (also known DNPI, Slc17a6)
- TH:
-
Midline and intralaminar thalamus
References
Berendse HW, Galis-de Graaf Y, Groenewegen HJ (1992) Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol 316:314–347
Bhatnagar S, Dallman MF (1999) The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res 851:66–75
Bhatnagar S, Viau V, Chu A, Soriano L, Meijer OC, Dallman MF (2000) A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci 20:5564–5573
Bubser M, Deutch AY (1998) Thalamic paraventricular nucleus neurons collateralize to innervate the prefrontal cortex and nucleus accumbens. Brain Res 787:304–310
Carr DB, Sesack SR (2000) Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci 20:3864–3873
DeFrance JF, Marchand JF, Sikes RW, Chronister RB, Hubbard JI (1985) Characterization of fimbria input to nucleus accumbens. J Neurophysiol 54:1553–1567
Deutch AY, Bubser M, Young CD (1998) Psychostimulant-induced Fos protein expression in the thalamic paraventricular nucleus. J Neurosci 18:10680–10687
Dobo E, Takacs VT, Gulyas AI, Nyiri G, Mihaly A, Freund TF (2011) New silver-gold intensification method of diaminobenzidine for double-labeling immunoelectron microscopy. J Histochem Cytochem 59:258–269
Fanselow MS, Dong, HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65:7–19
Floresco SB, West AR, Ash B, Moore H, Grace AA (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973
French SJ, Totterdell S (2002) Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol 446:151–165
French SJ, Totterdell S (2003) Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience 119:19–31
Fuller TA, Russchen FT, Price JL (1987) Sources of presumptive glutamergic/aspartergic afferents to the rat ventral striatopallidal region. J Comp Neurol 258:317–338
Gerfen CR, Sawchenko PE (1984) An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res 290:219–238
Goto Y, Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 8:805–812
Grace AA, Floresco SB, Goto Y, Lodge DJ (2007) Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci 30:220–227
Groenewegen HJ, Becker NE, Lohman AH (1980) Subcortical afferents of the nucleus accumbens septi in the cat, studied with retrograde axonal transport of horseradish peroxidase and bisbenzimid. Neuroscience 5:1903–1916
Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP (1987) Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 23:103–120
Groenewegen HJ, Berendse HW, Haber SN (1993) Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience 57:113–142
Haber SN, Groenewegen HJ, Grove EA, Nauta WJ (1985) Efferent connections of the ventral pallidum: evidence of a dual striato pallidofugal pathway. J Comp Neurol 235:322–335
Hartig W, Riedel A, Grosche J, Edwards RH, Fremeau RT Jr, Harkany T, Brauer K, Arendt T (2003) Complementary distribution of vesicular glutamate transporters 1 and 2 in the nucleus accumbens of rat: relationship to calretinin-containing extrinsic innervation and calbindin-immunoreactive neurons. J Comp Neurol 465:1–10
Heimer L, Zaborszky L, Zahm DS, Alheid GF (1987) The ventral striatopallidothalamic projection: I. The striatopallidal link originating in the striatal parts of the olfactory tubercle. J Comp Neurol 255:571–591
Ichinohe N, Hyde J, Matsushita A, Ohta K, Rockland KS (2008) Confocal mapping of cortical inputs onto identified pyramidal neurons. Front Biosci 13:6354–6373
Jackson ME, Frost AS, Moghaddam B (2001) Stimulation of prefrontal cortex at physiologically relevant frequencies inhibits dopamine release in the nucleus accumbens. J Neurochem 78:920–923
Jongen-Relo AL, Voorn P, Groenewegen HJ (1994) Immunohistochemical characterization of the shell and core territories of the nucleus accumbens in the rat. Eur J Neurosci 6:1255–1264
Kalivas PW, Churchill L, Klitenick MA (1993) GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience 57:1047–1060
Kaneko T, Fujiyama F, Hioki H (2002) Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol 444:39–62
Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311
Kelley AE, Domesick VB (1982) The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxidase study. Neuroscience 7:2321–2335
Koob GF, Bloom FE (1988) Cellular and molecular mechanisms of drug dependence. Science 242:715–723
McDonald AJ (1991) Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 44:1–14
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
O’Donnell P, Grace AA (1993) Physiological and morphological properties of accumbens core and shell neurons recorded in vitro. Synapse 13:135–160
O’Donnell P, Grace AA (1995) Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci 15:3622–3639
O’Donnell P, Grace AA (1998) Dysfunctions in multiple interrelated systems as the neurobiological bases of schizophrenic symptom clusters. Schizophr Bull 24:267–283
Overton PG, Tong ZY, Clark D (1996) A pharmacological analysis of the burst events induced in midbrain dopaminergic neurons by electrical stimulation of the prefrontal cortex in the rat. J Neural Transm 103:523–540
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, New York
Pinto A, Jankowski M, Sesack SR (2003) Projections from the paraventricular nucleus of the thalamus to the rat prefrontal cortex and nucleus accumbens shell: ultrastructural characteristics and spatial relationships with dopamine afferents. J Comp Neurol 459:142–155
Schultz W (2002) Getting formal with dopamine and reward. Neuron 36:241–263
Sloviter RS, Ali-Akbarian L, Horvath KD, Menkens KA (2001) Substance P receptor expression by inhibitory interneurons of the rat hippocampus: enhanced detection using improved immunocytochemical methods for the preservation and colocalization of GABA and other neuronal markers. J Comp Neurol 430:283–305
Tomioka R, Rockland KS (2006) Improved Golgi-like visualization in retrogradely projecting neurons after EGFP-adenovirus infection in adult rat and monkey. J Histochem Cytochem 54:539–548
Tunstall MJ, Oorschot DE, Kean A, Wickens JR (2002) Inhibitory interactions between spiny projection neurons in the rat striatum. J Neurophysiol 88:1263–1269
Usuda I, Tanaka K, Chiba T (1998) Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res 797:73–93
Wilson CJ, Chang HT, Kitai ST (1990) Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. J Neurosci 10:508–519
Wouterlood FG, Hrtig W (1995) Calretinin-immunoreactivity in mitral cells of the rat olfactory bulb. Brain Res 682:93–100
Wright CI, Groenewegen HJ (1996) Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience 73:359–373
Young CD, Deutch AY (1998) The effects of thalamic paraventricular nucleus lesions on cocaine-induced locomotor activity and sensitization. Pharmacol Biochem Behav 60:753–758
Acknowledgments
This work was supported by a NIH NS030549, NIH DA09158, EU GENADDICT LSHM-CT-2004-005166, NKTH-OTKA, CNK77793, HHMI 55005608 grants. The excellent technical assistance of E. Simon Szépné and Gy. Goda is also acknowledged. E. P. is a grantee of the János Bólyai scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papp, E., Borhegyi, Z., Tomioka, R. et al. Glutamatergic input from specific sources influences the nucleus accumbens-ventral pallidum information flow. Brain Struct Funct 217, 37–48 (2012). https://doi.org/10.1007/s00429-011-0331-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-011-0331-z