Abstract
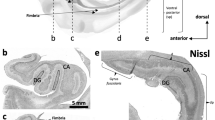
The cerebral cortex conveys major input to the granule cell layer of the cerebellar hemispheres by way of the pontine nuclei. Cerebrocortical projections terminate in multiple, widely distributed clusters in the pontine nuclei. This clustered organization is thought to provide the transition between the different organizational principles of the cerebrum and cerebellum, and indicates that parallel processing occurs at multiple sites in the pontine nuclei. At a cellular level, however, it is unknown whether individual cerebropontine neurons target pontocerebellar cells located in different clusters or not. We have employed anterograde axonal tracing and 3D computerized reconstruction techniques to characterize the branching pattern and morphology of individual cerebropontine axons from the primary somatosensory cortex (SI). Our findings show that 43% of the cerebrobulbar fibers arising from SI whisker representations provide two or three fibers entering the pontine nuclei, whereas 39% have only one fiber, and the remaining 18% do not project to the pontine nuclei. Thus, it appears that a majority of cerebropontine axons originating in SI whisker representations diverge to contact multiple, separated pontocerebellar cells. Further, 84% of the somatosensory cerebropontine fibers are collateral branches from cerebrobulbar and/or cerebrospinal parent fibers, while 16% are direct cerebropontine projections without a further descending projection. A range of thicknesses of the fibers entering the pontine nuclei were observed, with collaterals of corticobulbar fibers having the smallest diameter. Taken together, these findings may be related to previously described separate cerebropontine transmission lines with different properties.




Similar content being viewed by others
References
Allen GI, Korn H, Oshima T (1969) Monosynaptic pyramidal activation of pontine nuclei cells projecting to the cerebellum. Brain Res 15:272–275
Allen GI, Korn H, Oshima T, Toyama K (1975) The mode of synaptic linkage in the cerebro-ponto-cerebellar pathway of the cat. II. Responses of single cells in the pontine nuclei. Exp Brain Res 24:15–36
Bjaalie JG, Leergaard TB (2000) Functions of the pontine nuclei in cerebro-cerebellar communication. Trends Neurosci 23:152–153
Bjaalie JG, Leergaard TB (2006) Three-dimensional computerized reconstruction from serial sections: cell populations, regions, and whole brain. In: Zaborszky L, Wouterlood FG, Lanciego JL (eds) Neuroanatomical tract tracing: molecules, neurons, and systems. Springer Science+Business Media, New York, pp 530–565
Bjaalie JG, Leergaard TB, Lillehaug S, Odeh F, Moene IA, Kjode JO, Darin D (2005) Database and tools for analysis of topographic organization and map transformations in major projection systems of the brain. Neuroscience 136:681–695
Bower JM, Kassel J (1990) Variability in tactile projection patterns to cerebellar folia crus IIA of the Norway rat. J Comp Neurol 302:768–778
Bower JM, Beermann DH, Gibson JM, Shambes GM, Welker W (1981) Principles of organization of a cerebro-cerebellar circuit. Micromapping the projections from cerebral (SI) to cerebellar (granule cell layer) tactile areas of rats. Brain Behav Evol 18:1–18
Brevik A, Leergaard TB, Svanevik M, Bjaalie JG (2001) Three-dimensional computerised atlas of the rat brain stem precerebellar system: approaches for mapping, visualization, and comparison of spatial distribution data. Anat Embryol 204:319–332
Brodal P (1982) The cerebropontocerebellar pathway: salient features of its organization. In: Chan-Palay V, Palay S (eds) The cerebellum: new vistas. Exp Brain Res, suppl 6. Springer, Berlin, pp 108–132
Brodal P, Bjaalie JG (1992) Organization of the pontine nuclei. Neurosci Res 13:83–118
Brodal P, Bjaalie JG (1997) Salient anatomic features of the cortico-ponto-cerebellar pathway. Prog Brain Res 114:227–249
Brown LL (1992) Somatotopic organization in rat striatum: evidence for a combinational map. Proc Natl Acad Sci USA 89:7403–7407
Chapin JK, Lin CS (1984) Mapping the body representation in the SI cortex of anesthetized and awake rats. J Comp Neurol 229:199–213
Endo K, Araki T, Yagi N (1973) The distribution and pattern of axon branching of pyramidal tract cells. Brain Res 57:484–491
Fabri M, Burton H (1991) Topography of connections between primary somatosensory cortex and posterior complex in rat: a multiple fluorescent tracer study. Brain Res 538:351–357
Graybiel AM (1990) Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13:244–254
Hartmann MJ, Bower JM (2001) Tactile responses in the granule cell layer of cerebellar folium crus IIa of freely behaving rats. J Neurosci 21:3549–3563
Hoffer ZS, Arantes HB, Roth RL, Alloway KD (2005) Functional circuits mediating sensorimotor integration: quantitative comparisons of projections from rodent barrel cortex to primary motor cortex, neostriatum, superior colliculus, and the pons. J Comp Neurol 488:82–100
Holländer H, Brodal P, Walberg F (1968) Electronmicroscopic observations on the structure of the pontine nuclei and the mode of termination of the corticopontine fibres. An experimental study in the cat. Exp Brain Res 7:95–110
Houk JC, Mugnaini E (2003) Cerebellum. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ (eds) Fundamental neuroscience. Academic, San Diego, pp 841–872
Ito M (2000) Mechanisms of motor learning in the cerebellum. Brain Res 886:237–245
Ito M (2005) Bases and implications of learning in the cerebellum—adaptive control and internal model mechanism. Prog Brain Res 148:95–109
Lanciego JL, Wouterlood FG (1994) Dual anterograde axonal tracing with Phaseolus vulgaris-leucoagglutinin (PHA-L) and biotinylated dextran amine (BDA). Neuroscience Protocols 94-050-06-01-13
Leergaard TB (2003) Clustered and laminar topographic patterns in rat cerebro-pontine pathways. Anat Embryol 206:149–162
Leergaard TB, Alloway KD, Mutic JJ, Bjaalie JG (2000a) Three-dimensional topography of corticopontine projections from rat barrel cortex: correlations with corticostriatal organization. J Neurosci 20:8474–8484
Leergaard TB, Lyngstad KA, Thompson JH, Taeymans S, Vos BP, De Schutter E, Bower JM, Bjaalie JG (2000b) Rat somatosensory cerebropontocerebellar pathways: spatial relationships of the somatotopic map of the primary somatosensory cortex are preserved in a three-dimensional clustered pontine map. J Comp Neurol 422:246–266
Leergaard TB, Alloway KD, Pham TA, Bolstad I, Hoffer ZS, Pettersen C, Bjaalie JG (2004) Three-dimensional topography of corticopontine projections from rat sensorimotor cortex: comparisons with corticostriatal projections reveal diverse integrative organization. J Comp Neurol 478:306–322
Leergaard TB, Lillehaug S, De Schutter E, Bower JM, Bjaalie JG (2006) Topographical organization of pathways from somatosensory cortex through the pontine nuclei to tactile regions of the rat cerebellar hemispheres. Eur J Neurosci 24:2801–2812
Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC (1995) Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 15:1835–1853
Malach R (1994) Cortical columns as devices for maximizing neuronal diversity. Trends Neurosci 17:101–104
Mihailoff GA, McArdle CB, Adams CE (1981) The cytoarchitecture, cytology, and synaptic organization of the basilar pontine nuclei in the rat. I. Nissl and Golgi studies. J Comp Neurol 195:181–201
Möck M, Schwarz C, Thier P (1997) Electrophysiological properties of rat pontine nuclei neurons in vitro II. Postsynaptic potentials. J Neurophysiol 78:3338–3350
Moene IA, Subramaniam S, Darin D, Leergaard TB, Bjaalie JG (2007) Towards a workbench for rodent brain image data: systems architecture and design. Neuroinformatics 5:35–58
Odeh F, Ackerley R, Bjaalie JG, Apps R (2005) Pontine maps linking somatosensory and cerebellar cortices are in register with climbing fiber somatotopy. J Neurosci 25:5680–5690
Oshima T (1979) The microphysiology of pontine nulei in the cat concerning the concept of internal feedback. In: Massion J, Sasaki K (eds) Cerebro-cerebellar interactions. Elsevier, Amsterdam, pp 125–139
Palomero-Gallagher N, Zilles K (2004) Isocortex. In: Paxinos G (ed) The rat nervous system. Elsevier Academic Press, San Diego, pp 729–757
Pijpers A, Ruigrok TJ (2006) Organization of pontocerebellar projections to identified climbing fiber zones in the rat. J Comp Neurol 496:513–528
Ramón y Cajal S (1952) Histologie du Système Nerveux d l’Homme et des Vertébrés. Instituto Ramón y Cajal, Madrid
Reiner A, Veenman CL, Medina L, Jiao Y, Del Mar N, Honig MG (2000) Pathway tracing using biotinylated dextran amines. J Neurosci Methods 103:23–37
Rüegg DG, Sequin JJ, Wiesendanger M (1977) Effects of electrical stimulation of somatosensory and motor areas of the cerebral cortex on neurons of the pontine nuclei in squirrel monkeys. Neuroscience 2:923–927
Schmahmann JD (1997) Rediscovery of an early concept. Int Rev Neurobiol 41:3–27
Schmahmann JD (2004) Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 16:367–378
Schmahmann JD, Pandya DN (1997) The cerebrocerebellar system. Int Rev Neurobiol 41:31–60
Schwarz C, Möck M (2001) Spatial arrangement of cerebro-pontine terminals. J Comp Neurol 435:418–432
Schwarz C, Thier P (1995) Modular organization of the pontine nuclei: dendritic fields of identified pontine projection neurons in the rat respect the borders of cortical afferent fields. J Neurosci 15:3475–3489
Schwarz C, Thier P (1999) Binding of signals relevant for action: towards a hypothesis of the functional role of the pontine nuclei. Trends Neurosci 22:443–451
Shambes GM, Gibson JM, Welker W (1978) Fractured somatotopy in granule cell tactile areas of rat cerebellar hemispheres revealed by micromapping. Brain Behav Evol 15:94–140
Shumway CA, Morissette J, Gruen P, Bower JM (1999) Plasticity in cerebellar tactile maps in the adult rat. J Comp Neurol 413:583–592
Stanfield BB, O’Leary DD (1985) The transient corticospinal projection from the occipital cortex during the postnatal development of the rat. J Comp Neurol 238:236–248
Stein JF, Glickstein M (1992) Role of the cerebellum in visual guidance of movement. Physiol Rev 72:967–1017
Tomasch J (1968) The overall information carrying capacity of the major afferent and efferent cerebellar cell and fiber systems. Confin Neurol 30:359–367
Tomasch J (1969) The numerical capacity of the human cortico-ponto-cerebellar system. Brain Res 13:476–484
Torigoe Y, Blanks RHI, Precht W (1986) Anatomical studies on the nucleus reticularis tegmenti pontis in the pigmented rat. II. Subcortical afferents demonstrated by the retrograde transport of horseradish peroxidate. J Comp Neurol 243:88–105
Ugolini G, Kuypers HG (1986) Collaterals of corticospinal and pyramidal fibres to the pontine grey demonstrated by a new application of the fluorescent fibre labelling technique. Brain Res 365:211–227
Veenman CL, Reiner A, Honig MG (1992) Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J Neurosci Methods 41:239–254
Voogd J (1995) Cerebellum. In: Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ (eds) Gray’s anatomy. Churchill Livingstone, New York, pp 1027–1065
Welker C (1971) Microelectrode delineation of fine grain somatotopic organization of (SmI) cerebral neocortex in albino rat. Brain Res 26:259–275
Woolsey TA, Van der Loos H (1970) The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitectonic units. Brain Res 17:205–242
Acknowledgments
We thank Peter Szucs, Francis Odeh, and Paul Johannes Helm for valuable advice concerning electrophysiological mapping, iontophoretic injections, and microscopy, and Christian Pettersen, Dagmar Magalei, and Anna Torbjørg Bore for expert technical assistance. This project was funded by The Research Council of Norway.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bolstad, I., Leergaard, T.B. & Bjaalie, J.G. Branching of individual somatosensory cerebropontine axons in rat: evidence of divergence. Brain Struct Funct 212, 85–93 (2007). https://doi.org/10.1007/s00429-007-0145-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-007-0145-1