Abstract
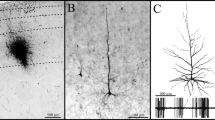
The optic tectum is reciprocally connected to the nuclei isthmi pars magnocellularis (Imc) and pars parvocellularis (Ipc), which have different modulatory effects on optic transmission. We studied the axon arbourisation of these isthmic nuclei in the optic tectum in order to differentiate between them using Golgi-impregnated preparations both in chickens and pigeons. In addition, sections from animals injected with the anterograde tracer biotinylated dextran-amine (BDA) into the Imc were examined in the bright-field and electron microscope to identify the axon arbourisations and terminals. Also, GABA immunogold stained sections were examined in the electron microscope. In Golgi preparations, slab-like (or poplar tree-like) axon terminal arbourisations of both magnocellular and parvocellular isthmic nuclei neurons were found extending to the tectal surface, with similar branching patterns, but different lengths. The axon arbourisations extending from layer 5 of the optic tectum to the surface were termed type 1, whereas those extending from the internal (12–11) layers to the tectal surface were termed type 2. Type 2 arbourisations very closely matched arbourisations observed in BDA injected material, indicating that Imc neurons gave rise to type 2 arbourisations. The two kinds of axon arbourisation in the external tectal layers were alike in both types of bird, except for the width, which was about 10 μm larger in the type 2 axon arbour. Controlling for size, there was no significant difference between chicks and pigeons. The significance of these afferents in the optic tectum is discussed.









Similar content being viewed by others
References
Angaut P, Repérant J (1976) Fine structure of optic fibre termination layers in pigeon optic tectum: a Golgi and electron microscope study. Neuroscience 1:93–105
Bagnoli P, Fontanesi GA, Streit P, Domenici L, Alesi R (1989) Changing distribution of GABA-like immunoreactivity in pigeon visual areas during the early posthatching period and effects of tectal removal on tectal GABAergic system. Vis Neurosci 3:491–508
Domenici L, Waldvogel HJ, Matute C, Streit P (1988) Distribution of GABA-like immunoreactivity in pigeon brain. Neuroscience 25:931–950
Felix D, Wu GY, Wang SR (1994) GABA as an inhibitory transmitter in the pigeon isthmo-tectal pathway. Neurosci Lett 169:212–214
Hardy O, Leresche N, Jassik-Gerschenfeld D (1982) The spatial organization of the excitatory regions in the visual receptive fields of pigeon’s optic tectum. Exp Brain Res 46:59–68
Hardy O, Leresche N, Jassik-Gerschenfeld D (1984) Postsynaptic potentials in neurons of the pigeon’s optic tectum in response to afferent stimulation from the retina and other visual structures: an intracellular study. Brain Res 311:65–74
Hardy O, Leresche N, Jassik Gerschenfeld D (1985) Morphology and laminar distribution of electrophysiologically identified cells in pigeon’s optic tectum: an intracellular study. J Comp Neurol 233:390–404
Hayes BP, Webster KE (1975) An electron microscopic study of retino-receptor layers in the pigeon optic tectum. J Comp Neurol 162:447–466
Hunt SP, Künzle H (1976) Observation on the projection and intrinsic organisation of the pigeon tectum opticum: an autographic study based on anterograde and retrograde axonal flow. J Comp Neurol 170:153–172
Hunt SP, Streit P, Künzle H, Cuenod M (1977) Characterization of the pigeon isthmo-tectal uptake and retrograde movement of radioactive compounds and by Golgi-like horseradish peroxidase labeling. Brain Res 129:197–212
Karten HJ (1965) Projection of the optic tectum of the pigeon (Columba livia). Anat Rec 151:369
Kuenzel WJ, Masson M (1988) A stereotaxic atlas of the brain of the chick (Gallus domesticus). The Johns Hopkins University Press, Baltimore London
McBain CJ, Fisahn A (2001) Interneurons unbound. Nat Rev Neurosci 2:11-23
Mott DD, Dingledine R (2003) Interneuron diversity series: interneuron research—challenges and strategies. Trends Neurosci 2003 26:484-488
O’Flaherty JJ (1971) A Golgi analysis of the tectum opticum of the Mallard duck. J Hirnforsch 12:389–404
Ramón y Cajal S (1911) Histologie du systeme nerveux de l’homme et des vertebres (translated by L. Azoulay) vol II. Maloine, Paris, pp 196–212
Repérant J, Angaut P (1977) The retinotectal projections in the pigeon. An experimental optical and electron microscope study. Neuroscience 2:119–140
Sebestény T, Davies DC, Zayats N, Németh A, Tömböl T (2002) The ramification and connections of retinal fibres in layer 7 of the domestic chick optic tectum. A Golgi impregnation, anterograde tracer and GABA-immunogold study. J Anat 200:169–183
Somogyi P, Hudgson AJ (1985) Antiserum to amino-butiric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections in cat striate cortex. J Histochem Cytochem 33:249–257
Stone J, Freeman JA (1971) Synaptic organisation of pigeon optic tectum: a Golgi and current-source density analysis. Brain Res 27:203–221
Tömböl T (1998) Golgi and electron-microscopic Golgi-GABA immunostaining study of the avian optic tectum. Acta Anat 162:209–255
Tömböl T, Egedy Gy, Németh A (1995) Some data on connections of neurons of nuclei isthmi of the chicken. J Brain Res 36:501–508
Tömböl T, Németh A (1998) Golgi and electron-microscopic Golgi-GABA immunostaining study of the avian optic tectum. Acta Anat 162:209–225
Tömböl T, Németh A (1999) Direct connections between dendritic terminals of tectal ganglion cells and glutamate-positive terminals of presumed optic fibres in layers 4–5 of the optic tectum of Gallus domesticus. A light- and electron microscopic study. Neurobiology (Budapest) 7:45–66
Tömböl T, Eyre M, Zayats N, Németh A (2003) The ramification and terminals of optic fibres in layers 2 and 3 of avian optic tectum: a Golgi and light and electron microscopic anterograde tracer study. Cells Tissue Organs 175:202–222
Valverde F (1962) Intrinsic organizations of the amygdaloid complex. Trab Inst Cajal Invest Biol 54:291–314
Wang SR (2003) The nucleus isthmi and dual modulation of the receptive field of tectal neurons in non-mammals. Brain Res Rev 41:13–25
Wang SR, Wang YC, Frost BJ (1995a) Magnocellular and parvocellular divisions of pigeon nucleus isthmi differentially modulate visual responses in the tectum. Exp Brain Res 194:376–384
Wang SR, Wu GY, Felix D (1995b) Avian Imc-tectal projection is mediated by acetylcholine and glutamate. Neuroreport 6:757–760
Wang YC, Frost BJ (1991) Visual response characteristics of neurons in nucleus isthmi mgnocellularis and nucleus isthmi parvocellularis of pigeons. Exp Brain Res 87:624–633
Wang Y, Xiao J, Wang SR (2000) Excitatory and inhibitory receptive fields of tectal cells are differentially modified by magnocellular and parvocellular divisions of the pigeon nucleus isthmi. J Comp Physiol A 186:505–511
Wang Y, Major DE, Karten HJ (2004) Morphology and connections of nuclei isthmi pars magnocellularis in chicks (Gallus gallus). J Comp Neurol 469:275–297
Wu GY, Wang SR, Felix D (1994) Effect of acetylcholine and NMDA on neurons of avian tectum and nucleus isthmi. Neuroreport 5:850–852
Acknowledgements
The authors express their grateful thanks to Jozsef Kiss for the fine micrographs. This work was supported by Grant T03725
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tömböl, T., Eyre, M.D., Alpár, A. et al. The axon arbourisation of nuclei isthmi neurons in the optic tectum of the chick and pigeon. A Golgi and anterograde tracer-study. Anat Embryol 209, 371–380 (2005). https://doi.org/10.1007/s00429-004-0450-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-004-0450-x