Abstract
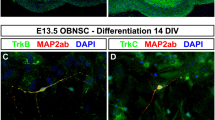
We previously established a primary culture system of the accessory olfactory bulb (AOB) to investigate the functional roles of individual types of neuron in pheromonal signal processing. However, the detailed characteristics of cultured AOB neurons were not yet apparent. In the present study, we address the cytological aspects of cultured AOB neurons using immunocytochemical staining methods. Cultured AOB neurons were compared with cultured main olfactory bulb (MOB) neurons in neuronal composition, maturational time course, and cell size. The number of total neurons, measured by microtubule-associated protein 2 (MAP2) immunostaining, progressively decreased, and glutamic acid decarboxylase positive (GAD+) interneurons were scarcely changed in their number in both AOB and MOB cultures over the culture periods. In contrast, the number of tyrosine hydroxylase positive (TH+) neurons in AOB cultures showed a slight, but significant, increase over time in culture, while those in MOB cultures remarkably decreased. The numbers of total neurons and GAD+ neurons were significantly greater in AOB cultures than in MOB cultures at all investigated time points. However, the numbers of TH+ neurons were lower at 7 days in vitro (DIV) and greater at 21 DIV in AOB cultures than in MOB cultures. The somatic sizes of all types of neurons at 14 DIV were significantly larger in AOB cultures than in MOB cultures. Furthermore, the frequency distributions of somatic sizes of total, GAD+, and TH+ neurons were significantly different between AOB and MOB cultures. These subtle differences in vitro may reflect in vivo differences between the AOB and MOB.



Similar content being viewed by others
References
Baker H (1986) Species differences in the distribution of substance P and tyrosine hydroxylase immunoreactivity in the olfactory bulb. J Comp Neurol 252:206–226
Baker H, Liu N, Chun HS, Saino S, Berlin R, Volpe B, Son JH (2001) Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb. J Neurosci 21:8505–8513
Bayer SA (1983) 3H-Thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp Brain Res 50:329–340
Davis BJ, Macrides F (1983) Tyrosine hydroxylase immunoreactive neurons and fibers in the olfactory system of the hamster. J. Comp. Neurol 214:427–440
Halász N (1990) The vertebrate olfactory system, Akadémiai Kiadó, Budapest
Halász N, Ljungdahl A, Hökfelt T, Johansen O, Goldstein M, Park DH, Biberfield P (1977) Transmitter histochemistry of the rat olfactory bulb, I: immunohistochemical localization of monoamine synthesizing enzymes—support for intrabulbar, periglomerular dopamine neurons. Brain Res 126:455–474
Halász N, Johansen O, Hökfelt T, Ljungdahl A, Goldstein M (1981) Immunohistochemical identification of two types of dopamine neuron in the rat olfactory bulb as seen by serial sectioning. J Neurocytol 10:251–259
Hinds JW (1968a) Autoradiographic study of histogenesis in the mouse olfactory bulb, I: time of origin of neurons and neuroglia. J Comp Neurol 134:287–304
Hinds JW (1968b) Autoradiographic study of histogenesis in the mouse olfactory bulb, II: cell proliferation and migration. J Comp Neurol 134:305–322
Humphrey T (1940) The development of the olfactory and the accessory olfactory formations in human embryos and fetuses. J Comp Neurol 73:431–468
Kosaka T, Hataguchi Y, Hama K, Nagatsu I, Wu J-Y (1985) Coexistence of immunoreactivities for glutamate decarboxylase and tyrosine hydroxylase in some neurons in the periglomerular region of the rat main olfactory bulb: possible coexistence of gamma-aminobutyric acid (GABA) and dopamine. Brain Res 343:166–171
Matsutani S, Senba E, Tohyama M (1988) Neuropeptide- and neurotransmitter-related immunoreactivities in the developing rat olfactory bulb. J Comp Neurol 272:331–342
McLean JH, Shipley MT (1988) Postmitotic, postmigrational expression of tyrosine hydroxylase in olfactory bulb dopaminergic neurons. J Neurosci 8:3658–3669
Meisami E, Bhatnagar KP (1998) Structure and diversity in mammalian accessory olfactory bulb. Microscopy Res Tech 43:476–499
Mori K (1987) Membrane and synaptic properties of identified neurons in the olfactory bulb. Prog Neurobiol 29:275–320
Mugnaini E, Oertel WH, Wouterlood FF (1984) Immunocytochemical localization of GABA neurons and dopamine neurons in the rat main and accessory olfactory bulbs. Neurosci Lett 47:221–226
Muramoto K, Kato M, Matsuoka M, Kuroda Y, Ichikawa M (2001) A primary culture system of rat olfactory bulb forming many synapses similar to intact ones and spontaneously generating synchronous intracellular calcium oscillations. Anat Embryol 203:9–21
Muramoto K, Osada T, Kato-Negishi M, Kuroda Y, Ichikawa M (2003) Increase in the number of tyrosine hydroxylase-containing neurons in a primary culture system of the rat accessory olfactory bulb by co-culture with vomeronasal pockets. Neuroscience 116:985–994
Osada T, Ikai A, Costanzo RM, Matsuoka M, Ichikawa M (1999) Continual neurogenesis of vomeronasal neurons in vitro. J Neurobiol 40:226–233
Puche AC, Shipley MT (1999) Odor-induced, activity-dependent transneuronal gene induction in vitro: mediation by NMDA receptors. J Neurosci 19:1359–1370
Shepherd GM (1972) Synaptic organization of the mammalian olfactory bulb. Physiol Rev 52:864–917
Shepherd GM, Greer CA (1998) Olfactory bulb. In: Shepherd GM (ed) The synaptic organization of the brain, 4th edn. Oxford University Press, New York, pp 159–203
Smith CG (1935) The change in volume of the olfactory and accessory olfactory bulbs of the albino rat during postnatal life. J Comp Neurol 61:477–508
Specht LA, Pickel VM, Joh TH, Reis DJ (1981) Light-microscopic immunocytochemical localization of tyrosine hydroxylase in prenatal rat brain, II: late ontogeny. J Comp Neurol 199:255–276
Takami S, Graziadei PP (1992) The morphology of GABA-immunoreactive neurons in the accessory olfactory bulb of rats. Brain Res 588:317–323
Acknowledgements
This work was supported by a grant from Core Research for Evolutional Science and Technology (CREST) of the Japan Science and Technology Corporation (JST).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muramoto, K., Kato-Negishi, M., Kuroda, Y. et al. Differences in development and cellular composition between neuronal cultures of rat accessory and main olfactory bulbs. Anat Embryol 209, 129–136 (2004). https://doi.org/10.1007/s00429-004-0432-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-004-0432-z