Abstract
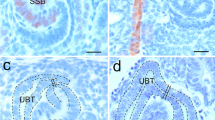
We aimed to define, for the first time, the ontogeny of intrarenal innervation and to assess the distribution and nature of parenchymal nerves in the human fetal kidney. Our material consisted of routinely-processed renal tissue sections from 17 human fetuses, six of 20–24 gestational weeks (gw) and 11 of 25–40 gw, and three adults. We used immunohistochemistry with antibodies to the pan-neural markers neuron-specific enolase (NSE), neurofilaments (NF), PGP9.5, S100, and the adrenergic marker tyrosine hydroxylase (TH). NSE-, NF-, S100-, and PGP9.5-positive nerves, associated with arterial and venous vasculature, were identified in the renal cortex from 20 gw onwards, and their density appeared to increase with gestation, reaching adult levels at 28 gw. Most of the intrarenal nerves were TH-positive. Nerve fibers extended from the corticomedullary region to the outer cortex, reaching the renal capsule in the 3rd trimester. In detail, NSE-, NF-, S100-, PGP9.5-, and TH-immunoreactive fibers were observed in close apposition to the renal artery and its branches, occasionally reaching the afferent and efferent arteriole (3rd trimester). Nerve fibers were detected in close apposition to the juxtaglomerular apparatus in the 2nd and 3rd trimesters. In the renal medulla, NSE-, PGP9.5-, S100-, and TH-positive nerve fibers were detected close to tubular cells as early as 20 gw. However, their density gradually decreased during the 3rd trimester, and they were not observed in the medulla of the adult kidney. In conclusion, the human fetal kidney appears richly innervated during the 2nd and 3rd trimesters. There is a progressive increase in the density of parenchymal nerve fibers towards term from the corticomedullary region to the cortex. Most intrarenal nerves are adrenergic and have a predominant perivascular distribution, implying that renal innervation plays an important functional role during intrauterine life.







Similar content being viewed by others
References
Ballesta J, Polak JM, Allen JM, Bloom SR (1984) The nerves of the juxtaglomerular apparatus of man and other mammals contain the potent peptide NPY. Histochemistry 80:483–485
Barajas L, Liu L (1993) The renal nerves of the newborn rat. Pediatr Nephrol 7:657–666
Barajas L, Muller J (1973) The innervation of the juxtaglomerular apparatus and surrounding tubules: a quantitative analysis by serial section electron microscopy. J Ultrastruct Res 43:107–132
Barajas L, Powers K, Wang P (1984) Innervation of the renal cortical tubules: a quantitative study. Am J Physiol 247:F50-F60
Bell C, Bhathal PS, Mann R, Ryan GB (1989) Evidence that dopaminergic sympathetic axons supply the medullary arterioles of human kidney. Histochemistry 91:361–364
Di Bona GF (1982) The functions of the renal nerves. Rev Physiol Biochem Pharmacol 94:176–181
Di Bona GF (2000) Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol 279:R1517-R1524
Di Bona GF (2003) Neural control of the kidney: past, present, and future. Hypertension 2:621–624
Ferguson M, Ryan GB, Bell C (1988) The innervation of the renal cortex in the dog. Cell Tissue Res 253:539–546
Gray JA, Kavlock RJ, Seidler FJ, Slotkin TA (1991) Neural factors in the development of renal function—effect of neonatal central catecholaminergic lesions with 6-hydroxydopamine. J Dev Physiol 15:325–330
Haimoto H, Takashi M, Koshikawa T, Asai J, Kato K (1986) Enolase isoenzymes in renal tubules and renal cell carcinoma. Am J Pathol 124:488–495
Liu L, Barajas L (1992) Prenatal and postnatal development of the renal innervation in the rat. J Am Soc Nephrol 3:566
Liu L, Barajas L (1993) The rat renal nerves during development. Anat Embryol 188:345–361
Lumbers ER, Yu ZY, Gibson KJ (2001) The selfish brain and the barker hypothesis. Clin Exp Pharmacol Physiol 28:942–947
Maretta M, Marettova E (1999) Immunohistochemical demonstration of vimentin and S-100 protein in the kidneys. Gen Physiol Biophys 18:100–102
Mata M, Alessi D, Fink DJ (1990) S100 is preferentially distributed in myelin-forming Schwann cells. J Neurocytol 19:432–442
McLachlan EM, Luff SE (1992) Sympathetic innervation of renal and extra-renal arterial vessels. Kidney Int 41:S56-S60
Mitchell GAG (1953) Anatomy of the autonomic nervous system. Churchill Livingstone, Edinburgh
Nolte C, Moos M, Schachner M (1999) Immunolocalization of the neural cell adhesion molecule L1 in epithelia of rodents. Cell Tissue Res 298:261–273
Page WV, Perlman S, Smith FG, Segar JL, Robillard JE (1992) Renal nerves modulate kidney renin gene expression during the transition from fetal to newborn life. Am J Physiol 262:R459-R463
Pupilli C, Gomez RA, Tuttle JB, Peach MJ, Carey RM (1991) Spatial association of renin-containing cells and nerve fibers in developing rat kidney. Pediatr Nephrol 5:690–695
Robillard JE, Guillery EN, Segar JL, Merrill DC, Jose PA (1993) Influence of renal nerves on renal function during development. Pediatr Nephrol 7(5):667–671
Sariola H, Ekblom P, Henke-Fahle S (1989) Embryonic neurons as in vitro inducers of differentiation of nephrogenic mesenchyme. J Dev Biol 132:271–281
Sainio K, Nonclercq D, Saarma M, Palgi J, Saxén L, Sariola H (1994) Neuronal characteristics in embryonic renal stroma. Int J Dev Biol 38:77–84
Shirato I, Asanuma K, Takeda Y, Hayashi K, Tomino Y (2000) Protein gene product 9.5 is selectively localized in parietal epithelial cells of Bowman’s capsule in the rat kidney. J Am Soc Nephrol 11:2381-2386
Slotkin TA, Lau C, Kavlock RJ, Gray JA, Orband-Miller L, Queen KL, et al. (1988a) Role of sympathetic neurons in biochemical and functional development of the kidney: neonatal sympathectomy with 6-hydroxydopamine. J Pharmacol Exp Ther 246:427–433
Slotkin TA, Levant B, Orband-Miller L, Queen KL, Stasheff S (1988b) Do sympathetic neurons coordinate cellular development in the heart and kidney? Effects of neonatal central and peripheral catecholaminergic lesions on cardiac and renal nucleic acids and proteins. J Pharmacol Exp Ther 244:166–172
Slotkin TA, Seidler FJ, Haim K, Cameron AM, Anolick L, Lau C (1988c) Neonatal central catecholaminergic lesions with intracisternal 6-hydroxydopamine: effects on development of presynaptic and postsynaptic components of peripheral sympathetic pathways and on the ornithine decarboxylase/polyamine system in heart, lung and kidney. J Pharmacol Exp Ther 247:975–982
Smith FG, Smith BA, Guillery EN, Robillard JE (1991) Role of the renal sympathetic nerves in lambs during the transition from fetal to newborn life. J Clin Invest 88:1988–1994
Smith FG, Klinkefus JM, Robillard JE (1992) Effects of volume expansion on renal sympathetic nerve activity, cardiovascular and renal function in lambs. Am J Physiol 262:R651-R658
Stevens A, Lowe JS (1997) Human histology. Mosby, London
Tainio H, Kylmala T, Heikkinen A (1992) Peptidergic innervation of the normal and obstructed human pyeloureteral junctions. Urol Int 48:31–34
Teratani T, Watanabe T, Yamahara K, Kumagai H, Ishikawa A, Arai K, Nozawa R (2002) Restricted expression of calcium-binding protein S100A5 in human kidney. Biochem Biophys Res Commun 291:623–627
Zimmermann HD (1972) Elektronenmikroskopische Befunde zur Innervation des Nephron nach Untersuchungen an der Fetalen Nachniere des Menschen. Zellforschung 129:65–75
Acknowledgements
We thank Dr. Stratigoula Sakellariou and Dr. Andriana Anagnostopoulou for their contribution in the selection of our material, and Dr. Aspassia Kyroudi-Voulgari for critical review of the manuscript. This study was financially supported by the Medical School and the ELKE Research Program “Kapodistrias” (70/4/6549), National and Kapodistrian University of Athens.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiniakos, D., Anagnostou, V., Stavrakis, S. et al. Ontogeny of intrinsic innervation in the human kidney. Anat Embryol 209, 41–47 (2004). https://doi.org/10.1007/s00429-004-0420-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-004-0420-3