Abstract
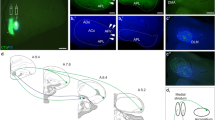
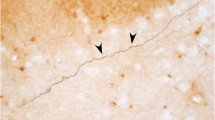
The amygdalohypothalamic projection, a major component of the stria terminalis, is involved in the conduction of emotional and olfactory information integrated in the amygdala to the hypothalamus to elicit emotional reactions. Despite the extensive studies on functional aspects of the amygdaloid complex, developmental mechanisms of the amygdala and related structures are still poorly understood. To investigate the development of the amygdalohypothalamic projection in the mouse embryonic brain, carbocyanine dye was applied to the amygdala to label the growing axons anterogradely and to the hypothalamus to label the amygdaloid neurons retrogradely. The initial outgrowth of the stria terminalis was found to be as early as E11.5. The pathway crossed in a saddle over the internal capsule, another prominent connection in the developing forebrain of the mammalian embryo. Bipolar immature neurons were distributed along the stria terminalis at the telencephalo-diencephalic boundary, and the internal capsule was also surrounded by these cells. These cells expressed immunoreactivities to calretinin and the lot-1 antigen which has been shown to be involved in guidance of the developing lateral olfactory tract. Ultrastructural analysis revealed an adherens-like junction between the stria terminalis and the apposed cells, implying contact-mediated guidance. These results suggest that, in the development of the stria terminalis, the axonal outgrowth is guided by a mechanism similar to that of the developing lateral olfactory tract, a major amygdalopetal connection.










Similar content being viewed by others
References
Abbott LC, Jacobowitz DM (1999) Developmental expression of calretinin-immunoreactivity in the thalamic eminence of the fetal mouse. Int J Dev Neurosci 17:331–345
Al-Shamma HA, De Vries GJ (1996) Neurogenesis of the sexually dimorphic vasopressin cells of the bed nucleus of the stria terminalis and amygdala of rats. J Neurobiol 29:91–98
Alheid GF, Beltramino CA, De Olmos JS, Forbes MS, Swanson DJ, Heimer L (1998) The neuronal organization of the supracapsular part of the stria terminalis in the rat: the dorsal component of the extended amygdala. Neuroscience 84:967–996
Amaral DG, Veazey RB, Cowan WM (1982) Some observations on hypothalamo-amygdaloid connections in the monkey. Brain Res 252:13–27
Angevine JB Jr (1970) Time of neuron origin in the diencephalon of the mouse. An autoradiographic study. J Comp Neurol 139:129–187
Auladell C, Martinez A, Alcantara S, Super H, Soriano E (1995) Migrating neurons in the developing cerebral cortex of the mouse send callosal axons. Neuroscience 64:1091–1103
Auladell C, Perez-Sust P, Super H, Soriano E (2000) The early development of thalamocortical and corticothalamic projections in the mouse. Anat Embryol 201:169–179
Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M (2002) Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 33:233–248
Bayer SA (1980) Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. J Comp Neurol 194:845–875
Bayer SA (1987) Neurogenetic and morphogenetic heterogeneity in the bed nucleus of the stria terminalis. J Comp Neurol 265:47–64
Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20:84–91
Berkelbach van der Sprenkel H (1926) Stria terminalis and amygdala in the brain of the opossum (Didelphis virginiana). J Comp Neurol 42:211–254
Bruce LL, Neary TJ (1995) The limbic system of tetrapods: a comparative analysis of cortical and amygdalar populations. Brain Behav Evol 46:224–234
Dani JW, Armstrong DM, Benowitz LI (1991) Mapping the development of the rat brain by GAP-43 immunocytochemistry. Neuroscience 40:277–287
De Olmos JS, Ingram WR (1972) The projection field of the stria terminalis in the rat brain. An experimental study. J Comp Neurol 146:303–334
Del Rio JA, Heimrich B, Borrell V, Forster E, Drakew A, Alcantara S, Nakajima K, Miyata T, Ogawa M, Mikoshiba K, Derer P, Frotscher M, Soriano E (1997) A role for Cajal-Retzius cells and reelin in the development of hippocampal connections. Nature 385:70–74
Easter SS Jr, Ross LS, Frankfurter A (1993) Initial tract formation in the mouse brain. J Neurosci 13:285–299
Ghosh A, Antonini A, McConnell SK, Shatz CJ (1990) Requirement for subplate neurons in the formation of thalamocortical connections. Nature 347:179–181
Han TM, De Vries GJ (1999) Neurogenesis of galanin cells in the bed nucleus of the stria terminalis and centromedial amygdala in rats: a model for sexual differentiation of neuronal phenotype. J Neurobiol 38:491–498
Hankin MH, Silver J (1988) Development of intersecting CNS fiber tracts: the corpus callosum and its perforating fiber pathway. J Comp Neurol 272:177–190
Heimer L, de Olmos J, Alheid GF, Zaborszky L (1991) “Perestroika” in the basal forebrain: opening the border between neurology and psychiatry. Prog Brain Res 87:109–165
Heimer L, Nauta WJ (1969) The hypothalamic distribution of the stria terminalis in the rat. Brain Res 13:284–297
Hirata T, Fujisawa H (1999) Environmental control of collateral branching and target invasion of mitral cell axons during development. J Neurobiol 38:93–104
Inoue T, Osatake H (1988) A new drying method of biological specimens for scanning electron microscopy: the t-butyl alcohol freeze-drying method. Arch Histol Cytol 51:53–59
Ivanova A, Yuasa S (1998) Neuronal migration and differentiation in the development of the mouse dorsal cochlear nucleus. Dev Neurosci 20:495–511
Johnston JB (1923) Further contributions to the study of the evolution of the forebrain. J Comp Neurol 35:337–481
Jurkevich A, Barth SW, Kuenzel WJ, Kohler A, Grossmann R (1999) Development of sexually dimorphic vasotocinergic system in the bed nucleus of stria terminalis in chickens. J Comp Neurol 408:46–60
Krettek JE, Price JL (1978) Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol 178:225–254
Lanuza E, Font C, Martinez-Marcos A, Martinez-Garcia F (1997) Amygdalo-hypothalamic projections in the lizard Podarcis hispanica: a combined anterograde and retrograde tracing study. J Comp Neurol 384:537–555
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184
Letinic K, Kostovic I (1997) Transient fetal structure, the gangliothalamic body, connects telencephalic germinal zone with all thalamic regions in the developing human brain. J Comp Neurol 384:373–395
Letinic K, Rakic P (2001) Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci 4:931–936
Ljungdahl A, Hokfelt T, Nilsson G (1978) Distribution of substance P-like immunoreactivity in the central nervous system of the rat-I. Cell bodies and nerve terminals. Neuroscience 3:861–943
Marin O, Baker J, Puelles L, Rubenstein JL (2002) Patterning of the basal telencephalon and hypothalamus is essential for guidance of cortical projections. Development 129:761–773
Martinez-Garcia F, Olucha FE, Teruel V, Lorente MJ (1993) Fiber connections of the amygdaloid formation of the lizard Podarcis hispanica. Brain Behav Evol 41:156–162
McConnell J, Angevine JB Jr (1983) Time of neuron origin in the amygdaloid complex of the mouse. Brain Res 272:150–156
Metin C, Godement P (1996) The ganglionic eminence may be an intermediate target for corticofugal and thalamocortical axons. J Neurosci 16:3219–3235
Mitrofanis J, Baker GE (1993) Development of the thalamic reticular and perireticular nuclei in rats and their relationship to the course of growing corticofugal and corticopetal axons. J Comp Neurol 338:575–587
Molnar Z, Adams R, Blakemore C (1998A) Mechanisms underlying the early establishment of thalamocortical connections in the rat. J Neurosci 18:5723–5745
Molnar Z, Adams R, Goffinet AM, Blakemore C (1998B) The role of the first post-mitotic cortical cells in the development of thalamocortical innervation in the reeler mouse. J Neurosci 18:5746–5765
Ni L, Jonakait GM (1988) Development of substance P-containing neurons in the central nervous system in mice: an immunocytochemical study. J Comp Neurol 275:493–510
Pires-Neto MA, Braga-De-Souza S, Lent R (1998) Molecular tunnels and boundaries for growing axons in the anterior commissure of hamster embryos. J Comp Neurol 399:176–188
Price JL, Slotnick BM, Revial MF (1991) Olfactory projections to the hypothalamus. J Comp Neurol 306:447–461
Sakanaka M, Shiosaka S, Takatsuki K, Inagaki S, Takagi H, Senba E, Kawai Y, Matsuzaki T, Tohyama M (1981) Experimental immunohistochemical studies on the amygdalofugal peptidergic (substance P and somatostatin) fibers in the stria terminalis of the rat. Brain Res 221:231–242
Sato Y, Hirata T, Ogawa M, Fujisawa H (1998) Requirement for early-generated neurons recognized by monoclonal antibody lot-1 in the formation of lateral olfactory tract. J Neurosci 18:7800–7810
Schwob JE, Price JL (1984) The development of axonal connections in the central olfactory system of rats. J Comp Neurol 223:177–202
Shammah-Lagnado SJ, Beltramino CA, McDonald AJ, Miselis RR, Yang M, de Olmos J, Heimer L, Alheid GF (2000) Supracapsular bed nucleus of the stria terminalis contains central and medial extended amygdala elements: evidence from anterograde and retrograde tracing experiments in the rat. J Comp Neurol 422:533–555
Song DD, Harlan RE (1994) The development of enkephalin and substance P neurons in the basal ganglia: insights into neostriatal compartments and the extended amygdala. Brain Res Dev Brain Res 83:247–261
Sretavan DW, Pure E, Siegel MW, Reichardt LF (1995) Disruption of retinal axon ingrowth by ablation of embryonic mouse optic chiasm neurons. Science 269:98–101
Sugisaki N, Hirata T, Naruse I, Kawakami A, Kitsukawa T, Fujisawa H (1996) Positional cues that are strictly localized in the telencephalon induce preferential growth of mitral cell axons. J Neurobiol 29:127–137
Tomioka N, Osumi N, Sato Y, Inoue T, Nakamura S, Fujisawa H, Hirata T (2000) Neocortical origin and tangential migration of guidepost neurons in the lateral olfactory tract. J Neurosci 20:5802–5812
Tuttle R, Nakagawa Y, Johnson JE, O’Leary DD (1999) Defects in thalamocortical axon pathfinding correlate with altered cell domains in Mash-1-deficient mice. Development 126:1903–1916
Ulfig N, Setzer M, Bohl J (1998) Transient architectonic features in the basolateral amygdala of the human fetal brain. Acta Anat 163:99–112
Ulfig N, Setzer M, Bohl J (1999) Distribution of GAP-43-immunoreactive structures in the human fetal amygdala. Eur J Histochem 43:19–28
Ulfig N (2002) The ganglionic eminence – a putative intermediate target of amygdaloid connections. Dev Brain Res 139:313–318
Wahlsten D (1981) Prenatal schedule of appearance of mouse brain commissures. Brain Res 227:461–473
Yuasa S, Kawamura K, Kuwano R, Ono K (1996) Neuron-glia interrelations during migration of Purkinje cells in the mouse embryonic cerebellum. Int J Dev Neurosci 14:429–438
Yuasa S, Kitoh J, Kawamura K (1994) Interactions between growing thalamocortical afferent axons and the neocortical primordium in normal and reeler mutant mice. Anat Embryol 190:137–154
Acknowledgements
This study was supported in part by research grants to S.Y. from the Ministry of Health, Labor and Welfare of Japan, the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Futaba Memorial Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aizawa, H., Sato, Y., Maekawa, M. et al. Development of the amygdalohypothalamic projection in the mouse embryonic forebrain. Anat Embryol 208, 249–264 (2004). https://doi.org/10.1007/s00429-004-0399-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-004-0399-9