Abstract
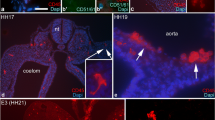
The origin of vimentin-positive secretory dendritic cells of the bursa of Fabricius was studied by chick–quail chimera, parabiosis and immunohistochemistry using species-specific monoclonal antibodies. Quail bursal primordia of different ages were transferred to coelomic cavity of 3-day-old chicken embryos and further incubated for 18 days. In transplanted quail bursas the secretory dendritic cells of chicken and quail origin were detected by double staining of vimentin plus 74.3 and vimentin plus QCPN monoclonal antibodies, respectively. In bursal primordia of 5- and 6-day-old quail embryos both dendritic cells and B cells were of host, i.e. chicken origin. Mixed dendritic cell population of quail and chick origin emerged in chimeric birds of 6.5 days of age. In quail embryos transplanted at 7 and 8 days of age both dendritic cells and B cells were mixed i.e. of chicken and quail origin. Bursal secretory dendritic cells and medullary epithelial cells create “dendro-epithelial tissue” to receive pre-B cells. Colonization of dendro-epithelial tissue by pre-B cells initiates at day 7, thus the colonization of bursal anlage by blood-borne cells is a two-step process; entering of dendritic cells at day 6.5 is followed by that of B cells at day 7 and afterwards. It is discussed that bursal secretory dendritic cells and their product are key elements of bursal function therefore the mammalian bursa equivalent organ might be represented by a cell, which is analogous with the bursal secretory dendritic cell.






Similar content being viewed by others
References
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN (2001) Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166:7556–7562
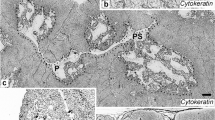
Bockman DE, Cooper MD (1973) Pinocytosis by epithelium associated with lymphoid follicles in the bursa of Fabricius, appendix, and Peyer’s patches. An electron microscopic study. Am J Anat 136:455–477
Boehmelt G, Madruga J, Dorfler P, Briegel K, Schwarz H, Enrietto PJ, Zenke M (1995) Dendritic cell progenitor is transformed by a conditional v-Rel estrogen receptor fusion protein v-RelER. Cell 80:341–352
Bofill M, Akbar AN, Amlot PL (2000) Follicular dendritic cells share a membrane-bound protein with fibroblasts. J Pathol 191:217–226
Boyden EA (1922) The development of the cloaca in birds, with special reference to the origin of the bursa of fabricius, the formation of a urodaeal sinus, and the regular occurrence of a cloacal fenestra. Am J Anat 30:1071–1074
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1:71–81
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM (2001) Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98:2396–2402
Ciriaco E, Garcia-Suarez O, Ricci A, Abbate F, Piedimonte G, Vega JA (1997) Trk-like proteins during the post-hatching growth of the avian bursa of Fabricius. Vet Immunol Immunopathol 55:313–320
Clark EA, Grabstein KH, Gown AM, Skelly M, Kaisho T, Hirano T, Shu GL (1995) Activation of B lymphocyte maturation by a human follicular dendritic cell line, FDC-1. J Immunol 155:545–555
Glick B (1995) Embryogenesis of the bursa of Fabricius: stem cell, microenvironment, and receptor-paracrine pathways. Poult Sci 74:419–426
Glick B, Oláh I (1984) A continuum of cells leading to an in vivo humoral response. Immunol Today 5:162–165
Glick B, Oláh I (1993) A bursal secretory dendritic cell and its contributions to the microenvironment of the developing bursal follicle. Res Immunol 144:446–448
Glick B, Chang TS, Jaap RG (1956) The bursa of Fabricius and antibody production in the domestic fowl. Poult Sci 35:224–225
Hamburger V, Hamilton HL (1951) A series of normal stages in the developing chick embryo. J Morphol 88:49–92
Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN (2001) Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 15:2215–2224
Humphrey JH, Grennan D, Sundaram V (1984) The origin of follicular dendritic cells in the mouse and the mechanism of trapping of immune complexes on them. Eur J Immunol 14:859–864
Houssaint E (1987) Cell lineage segregation during bursa of Fabricius ontogeny. J Immunol 138:3626–3634
Houssaint E, Belo M, Le Douarin NM (1976) Investigations on cell lineage and tissue interactions in the developing bursa of Fabricius through inter-specific chimeras. Dev Biol 53:250–264
Houssaint E, Diez E, Pink JR (1987) Ontogeny and tissue distribution of the chicken Bu-1a antigen. Immunol 62:463–470
Houssaint E, Lassila O, Vainio O (1989) Bu-1 antigen expression as a marker for B cell precursors in chicken embryos. Eur J Immunol 19:239–243
Jeurissen SH, Janse EM (1998) The use of chicken-specific antibodies in veterinary research involving three other avian species. Vet Q 20:140–143
Jeurissen SH, Janse EM, Ekino S, Nieuwenhuis P, Koch G, De Boer GF (1988) Monoclonal antibodies as probes for defining cellular subsets in the bone marrow, thymus, bursa of Fabricius, and spleen of the chicken. Vet Immunol Immunopathol 19:225–238
Jeurissen SH, Janse EM, Koch GL, De Boer GF (1989) Distribution and function of non-lymphoid cells positive for monoclonal antibody CVI-ChNL-68.2 in healthy chickens and those infected with Marek’s disease virus. Vet Immunol Immunopathol 22:123–133
Jeurissen SH, Claassen E, Janse EM (1992) Histological and functional differentiation of non-lymphoid cells in the chicken spleen. Immunol 77:75–80
Jeurissen SH, Janse EM, Lehrbach PR, Haddad EE, Avakian A, Whitfill CE (1998) The working mechanism of an immune complex vaccine that protects chickens against infectious bursal disease. Immunol 95:494–500
Kapasi ZF, Qin D, Kerr WG, Kosco-Vilbois MH, Shultz LD, Tew JG, Szakal AK (1998) Follicular dendritic cell (FDC) precursors in primary lymphoid tissues. J Immunol 160:1078–1084
Kaspers B, Lillehoj HS, Lillehoj EP 1993 Chicken macrophages and thrombocytes share a common cell surface antigen defined by a monoclonal antibody. Vet Immunol Immunopathol 36:333–346
Kincade PW, Lee G, Pietrangeli CE, Hayashi S, Gimble JM (1989) Cells and molecules that regulate B lymphopoiesis in bone marrow. Annu Rev Immunol 7:111–143
Kurz H, Christ B (1998) Embryonic CNS macrophages and microglia do not stem from circulating, but from extravascular precursors. Glia 22:98–102
Lupetti M, Dolfi A, Giannessi F, Bianchi F, Michelucci S (1990) Reappraisal of histogenesis in the bursal lymphoid follicle of the chicken. Am J Anat 187:287–302
Le Douarin NM, Houssaint E, Jotereau FV, Belo M (1975) Origin of hemopoietic stem cells in embryonic bursa of Fabricius and bone marrow studied through inter-specific chimeras. Proc Natl Acad Sci USA 72:2701–2705
Le Douarin NM, Dieterlen-Lievre F, Teillet MA (1996) Quail-chick transplantations. Methods Cell Biol 51:23–59
Mansikka A, Sandberg M, Lassila O, Toivanen P (1990) Rearrangement of immunoglobulin light chain genes in the chicken occurs prior to colonization of the embryonic bursa of Fabricius. Proc Natl Acad Sci USA 87:9416–9420
Masteller EL, Larsen RD, Carlson LM, Pickel JM, Nickoloff B, Lowe J, Thompson CB, Lee KP (1995) Chicken B cells undergo discrete developmental changes in surface carbohydrate structure that appear to play a role in directing lymphocyte migration during embryogenesis. Development 121:1657–1667
Masteller EL, Pharr GT, Funk PE, Thompson CB (1997) Avian B cell development. Int Rev Immunol 15:185–206
Mast J, Goddeeris BM, Peeters K, Vandesande F, Berghman LR (1998) Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet Immunol Immunopathol 61:343–357
McCormack WT, Tjoelker LW, Thompson CB (1991) Avian B-cell development: generation of an immunoglobulin repertoire by gene conversion. Annu Rev Immunol 9:219–241
Nagy N, Magyar A, David C, Gumati MK, Oláh I (2001) Development of the follicle-associated epithelium and the secretory dendritic cell in the bursa of Fabricius of the guinea fowl (Numida meleagris) studied by novel monoclonal antibodies. Anat Rec 262:279–292
van Nierop K, de Groot C (2002) Human follicular dendritic cells: function, origin and development. Semin Immunol 14:251–257
Oláh I, Glick B (1978) Secretory cell in the medulla of the bursa of Fabricius. Experientia 34:1642–1643
Oláh I, Glick B (1987) Bursal secretory cells: an electron microscope study. Anat Rec 219:268–274
Oláh I, Glick B, Törő I (1986) Bursal development in normal and testosterone-treated chick embryos. Poult Sci 65:574–588
Oláh I, Takács L, Törő I (1988) Formation of lymphoepithelial tissue in the sheep’s palatine tonsil. Acta Otolaryngol Suppl 454:7–17
Oláh I, Kendall C, Glick B (1992) Anti-vimentin monoclonal antibody recognizes a cell with dendritic appearance in the chicken’s bursa of Fabricius. Anat Rec 232:121–125
Oláh I, Gumati MK, Nagy N, Magyar A, Kaspers B, Lillehoj H (2002) Diverse expression of the K-1 antigen by cortico-medullary and reticular epithelial cells of the bursa of Fabricius in chicken and guinea fowl. Dev Comp Immunol 26:481–488
Otsubo Y, Chen N, Kajiwara E, Horiuchi H, Matsuda H, Furusawa S (2001) Role of bursin in the development of B lymphocytes in chicken embryonic bursa of Fabricius. Dev Comp Immunol 25:485–493
Pardanaud L, Dieterlen-Liévre F (1993) Emergence of endothelial and hemopoietic cells in the avian embryo. Anat Embryol 187:107–114
Pardanaud L, Altmann C, Kitos P, Dieterlen-Liévre F, Buck CA (1987) Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development 100:339–349
Pharr T, Oláh I, Bricker J, Olson WC, Ewert D, Marsh J, Glick B (1995) Characterization of a novel monoclonal antibody, EIV-E12, raised against enriched splenic ellipsoid-associated cells. Hybridoma 14:51–57
Pink JR, Rijnbeek AM (1983) Monoclonal antibodies against chicken lymphocyte surface antigens. Hybridoma 2:287–296
Pospisil R, Mage RG (1998) Rabbit appendix: a site of development and selection of the B cell repertoire. Curr Top Microbiol Immunol 229:59–70
Reynolds J (1997) The genesis, tutelage and exodus of B cells in the ileal Peyer’s patch of sheep. Int Rev Immunol 15:265–299
Romanoff AL (ed) (1960) The Avian Embryo: Structural and Functional Development. McMillan, New York
Sayegh CE, Demaries SL, Iacampo S, Ratcliffe MJ (1999) Development of B cells expressing surface immunoglobulin molecules that lack V(D)J-encoded determinants in the avian embryo bursa of fabricius. Proc Natl Acad Sci USA 96:10806–10811
Valinsky JE, Reich E, Le Douarin NM (1981) Plasminogen activator in the bursa of Fabricius: correlations with morphogenetic remodeling and cell migrations. Cell 25:471–476
Veromaa T, Vainio O, Eerola E, Toivanen P (1988) Monoclonal antibodies against chicken Bu-1a and Bu-1b alloantigens. Hybridoma 7:41–48
Weill JC, Reynaud CA (1987) The chicken B cell compartment. Science 238:1094–1098
Weill JC, Reynaud CA (1992) Early B-cell development in chickens, sheep and rabbits. Curr Opin Immunol 4:177–180
Acknowledgements
The authors wish to thank to Ildikó Schay for typing the manuscript, Dr. Sándor Paku for the technical assistance in confocal microscopy, Jutka Fügedi and Beáta Urák for laboratory and photographical assistance. This work was supported partly by OTKA, Grant number: T-042558, and partly by ETT, Grant number: 145/99.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagy, N., Magyar, A., Tóth, M. et al. Origin of the bursal secretory dendritic cell. Anat Embryol 208, 97–107 (2004). https://doi.org/10.1007/s00429-003-0378-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-003-0378-6