Abstract
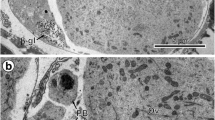
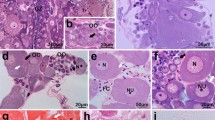
The formation of the egg envelope in a teleost, Dicentrarchus labrax (L.), was analysed at histological and ultrastructural level. The sequential deposition of three main layers (Z1, Z2 and Z3) constitutes the extracellular matrix throughout oocyte development. Various findings indicate that these subunits are biochemically distinct: (1) periodate- and phosphotungstic acid-reactive carbohydrates are obviously detected only in the Z1, that constitutes the initial deposit of the egg envelope in early lipidic oocytes; (2) a monoclonal antibody (DLE7) against egg envelope polypeptides did not immunostain the Z1 and the underlying Z2; (3) the antigenic determinants recognised by DLE7, thought to be exogenous in origin (synthesised in the liver), are incorporated in the inner layer (Z3). In addition, DLE7 immunostained a thin layer, assembled together with Z3. This line has not yet been described in teleost eggs and was named Z1a. This study first describes at fine cytological level the contribution of exogenous proteins to formation of the different egg envelope layers. Results obtained with conventional, immunochemical and cytochemical techniques suggest multiple synthetic sources (exogenous and follicular) of egg envelope proteins.






Similar content being viewed by others
Abbreviations
- IR :
-
Immunoreactivity
- LVS :
-
Lipid vesicle stage
- mAb :
-
Monoclonal antibody
- PAS :
-
Periodic acid–Schiff
- PBS :
-
Phosphate-buffered saline
- PTA :
-
Phosphotungstic acid
- TBS :
-
Tris-buffered saline
- TEM :
-
Transmission electron microscopy
- YGS :
-
Yolk granule stage
References
Abelli L, Gallo VP, Civinini A, Mastrolia L (1996) Immunohistochemical and ultrastructural evidence of adrenal chromaffin cell subtypes in sea bass Dicentrarchus labrax (L.). Gen Comp Endocrinol 102:113–122
Abraham M, Hilge V, Lison S, Tibika H (1984) The cellular envelope of oocytes in teleosts. Cell Tissue Res 235:403–410
Anderson E (1967) The formation of the primary envelope during oocyte differentiation in teleosts. J Cell Biol 35:193–212
Arukwe A, Knudsen FR, Goksøyr A (1997) Fish zona radiata (eggshell) protein: a sensitive biomarker for environmental estrogens. Environ Health Perspect 105(4):418–422
Arukwe A, Celius T, Walther BT, Goksøyr A (1998) Plasma levels of vitellogenin and eggshell zona radiata proteins in 4-nonylphenol- and o,p’-DDT treated juvenile Atlantic salmon (Salmo salar). Mar Environ Res 46:133–136
Arukwe A, Kullman SW, Hinton DE (2001) Differential biomarker gene and protein expressions in nonylphenol- and estradiol-17β-treated juvenile rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol C Toxicol Pharmacol 129(1):1–10
Arukwe A, Kullman SW, Berg K, Goksøyr A, Hinton DE (2002) Molecular cloning of rainbow trout (Oncorhynchus mykiss) eggshell zona radiata protein complementary DNA: mRNA expression in 17βestradiol- and nonylphenol-treated fish. Comp Biochem Physiol B Biochem Mol Biol 132(2):315–326
Baldacci A, Taddei AR, Mazzini M, Fausto AM, Buonocore F, Scapigliati G (2001) Ultrastructure and proteins of the egg chorion of the antarctic fish Chionodraco hamatus (Teleostei, Notothenioidei). Polar Biol 24:417–421
Barr WA (1968) Patterns of ovarian activity. In: Barrington EJW, Jørgensen CB (eds) Perspectives in endocrinology: hormones in the livers of lower vertebrates. Academic Press, New York, pp 164–238
Begovac PC, Wallace RA (1989) Major vitelline envelope proteins in pipefish oocytes originate within the follicle and are associated with the Z3 layer. J Exp Zool 251:56–73
Cawood AH, Potter U, Dickinson HG (1978) An evaluation of Coomassie brilliant blue as a stain for quantitative microdensitometry of protein in section. J Histochem Cytochem 26:645–650
Chang YS, Wang SC, Tsao CC, Huang FL (1996) Molecular cloning, structural analysis, and expression of carp ZP3 gene. Mol Reprod Dev 44:295–304
Chang YS, Hsu CC, Wang SC, Tsao CC, Huang FL (1997) Molecular cloning, structural analysis, and expression of carp ZP2 gene. Mol Reprod Dev 46:258–267
Davail B, Pakdel F, Bujo H, Perazzolo LM, Waclawek M, Schneider WJ, Le Menn F (1998) Evolution of oogenesis: the receptor for vitellogenin from the rainbow trout. J Lipid Res 39:1929–37
Del Giacco L, Vanoni C, Bonsignorio D, Duga S, Mosconi G, Santucci A Cotelli F (1998) Identification and spatial distribution of the mRNA encoding the gp49 component of the gilthead sea bream, Sparus aurata egg envelope. Mol Reprod Dev 49:58–69
Del Giacco L, Diani S, Cotelli F (2000) Identification and spatial distribution of the mRNA encoding an egg envelope component of the Cyprinid zebrafish, Danio rerio, homologous to the mammalian ZP3 (ZPC). Dev Genes Evol 210:41–46
Dumont JN, Brummett AR (1985) Egg envelopes in vertebrates. In: Browder LW (ed) Developmental biology, Oogenesis vol 1. Plenum Press, New York, pp 235–288
Fausto AM, Carcupino M, Scapigliati G, Taddei AR, Mazzini M (1994) Fine structure of the chorion and mycropyle of sea bass egg Dicentrarchus labrax (Linnaeus, 1758) (Teleostea, Percichthydae). Boll Zool 61:129–134
Fujita T, Shimizu M, Hiramatsu N, Fukada H, Hara A (2002) Purification of serum precursor protein to vitelline envelope (choriogenins) in masu salmon, Oncorhynchus masou. Comp Biochem Physiol B Biochem Mol Biol 132(3): 599–610
Guraya, SS (1986) The cell and molecular biology of fish oogenesis, follicular wall. Karger, Basel, pp 110–154
Hamazaki T, Iuchi I, Yamagami K (1985) A spawning female-specific substance reactive to anti-chorion (egg envelope) glicoprotein antibody in the teleost Oryzias latipes. J Exp Zool 235:269–279
Hamazaki TS, Iuchi I, Yamagami K (1987) Purification and identification of vitellogenin and its immunohistochemical detection in growing oocytes of the teleost, Oryzias latipes. J Exp Zool 242:333–341
Hart NH (1990) Fertilization in Teleost fishes: mechanism of sperm-egg interactions. Int Rev Cytol 121:1–66
Hosokawa K (1985) Electron microscopy observation of chorion formation in the teleost, Navadon modestus. Zool Sci 2:513–522
Hyllner SJ, Haux C (1992) Immunochemical detection of the major vitelline envelope proteins in the plasma and oocytes of the maturing female rainbow trout, Oncorhynchus mykiss. J Endocrinol 135:303–309
Hyllner SJ, Oppen-Berntsen DO, Helvik JV, Walther BT, Haux C (1991) Oestradiol-17β induces the major vitelline envelope protein in both sexes in teleosts. J Endocrinol 131:229–236
Hyllner SJ, Barber HFP, Larsson DJC, Haux C (1995) Amino acid composition and endocrine control of vitelline envelope proteins in the European sea bass (Dicentrarchus labrax) and gilthead sea bream (Sparus aurata). Mol Reprod Dev 41:339–347
Khoo KH (1979) The histochemistry and endocrine control of vitellogenesis in goldfish ovaries. Can J Zool 57:617–626
Ludwig H (1874) Gber die eibildung thierreiche. Arb Zool Zoot Inst. (Wurzburg) 1:287–510
Lyons CE, Payette KL, Price JL, Huang RCC (1993) Expression and structural analysis of a teleost homolog of mammalian zona pellucida gene. J Biol Chem 268:21351–21358
Mayer I, Shackley SE, Ryland JS (1988) Aspects of the reproductive biology of the bass, Dicentrarchus labrax L. I. A histological and histochemical study of oocytes development. J Fish Biol 33:609–622
Murata K, Iuchi I, Yamagami K (1994) Synchronous production of the low- and high-molecular weight precursors of the egg envelope subunits, in response to estrogen administration in the teleost fish Oryzias latipes. Gen Comp Endocrinol 95:232–239
Murata K, Sugiyama H, Yasumasu S, Iuchi I, Yasumasu I, Yamagami K (1997) Cloning of cDNA and estrogen-induced hepatic gene expression for choriogenin H, a precursor of the fish egg envelope (chorion). Proc Natl Acad Sci USA 94:2050–2055
Oppen-Berntsen DO, Helvik JV, Walther BT (1990) The major structural protein of cod (Gadus morhua) eggshells and protein cross linking during teleost egg hardening. Dev Biol 137:258–265
Pease DC (1970) Phosphotungstic acid as a specific electron stain for complex carbohydrates. J Histochem Cytochem 18:455–458
Perazzolo LM, Coward K, Davail B, Normand E, Tyler CR, Pakdel F, Schneider WJ, Le Menn F (1999) Expression and localization of messenger ribonucleic acid for the vitellogenin receptor in ovarian follicles throughout oogenesis in the rainbow trout, Oncorhynchus mykiss. Biol Reprod 60:1057–1068
Picchietti S, Taddei AR, Scapigliati G, Buonocore F, Fausto AM, Romano N, Mazzini M, Mastrolia L, Abelli L (2004) Immunoglobulin protein and gene transcripts in ovarian follicles throughout oogenesis in the teleost Dicentrarchus labrax (L.). Cell Tissue Res 315(2):259–270
Rambourg MA (1967) Détection des glycoprotéines en microscopie électronique: coloration de la surface cellulaire et de l’appareil de Golgi par un mélange acide cromique-phosphotungstique. CR Acad Sci Paris D 265:1426–1428
Ravaglia MA, Maggese MC (2003) Ovarian follicle ultrastructure in the teleost Synbranchus marmoratus (Bloch, 1795), with special reference to the vitelline envelope development. Tissue Cell 35(1):9–17
Robinson CD, Brown E, Craft JA, Davies IM, Moffat F, Pirie D, Robertson F, Stagg RM, Struthers S (2003) Effect of sewage effluent and ethynyl oestradiol upon molecular markers of oestrogenic exposure, maturation and reproductive success in the sand goby (Pomatoschistus minutus, Pallas). Aquat Toxicol 62:119–134
Scapigliati G, Carcupino M, Taddei AR, Mazzini M (1994) Characterization of the main egg envelope proteins of the sea bass Dicentrarchus labrax L. (Teleostea, Serranidae). Mol Reprod Dev 38:48–53
Scapigliati G, Meloni S, Mazzini M (1999) A monoclonal antibody against chorion proteins of the sea bass Dicentrarchus labrax (Linnaeus, 1758): studies of chorion precursors and applicability in immunoassays. Biol Reprod 60:783–789
Tesoriero JV (1977) Formation of the chorion (zona pellucida) in the teleost, Oryzias latipes. II. Polysaccharide cytochemistry of early oogenesis. J Histochem Cytochem 25:1376–1380
Thiéry JP (1967) Mise en evidence des polysaccharides sur coupes fines en microscopie électronique. J Microscopie (Paris) 6:987–1018
Tokarz RR (1978) Oogonial proliferation, oogenesis, and folliculogenesis in nonmammalian vertebrates. In: Jones RE (ed) The vertebrate ovary. Plenum Press, New York, pp 145–179
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte growth in teleost. Am Zool 21:325–343
Wang H, Gong Z (1999). Characterization of two zebra fish cDNA clones encoding egg envelope proteins ZP2 and ZP3. Biochem Biophys Acta 1446:156–160
Yamagami K, Hamazaki TS, Yasumasu S, Masuda K, Iuchi I (1992) Molecular and cellular basis of formation, hardening, and breakdown of the egg envelope in fish. Int Rev Cytol 136:51–92
Yamagami K (1996) Studies on the hatching enzyme (choriolysin) and its substrate, egg envelope, constructed of the precursors (choriogenins) in Oryzias latipes: a sequel to the information in 1991/1992. Zool Sci 13:331–340
Acknowledgements
We are grateful to Dr. Claudia Narduzzi for technical help. The authors greatly acknowledge Dr. Maria Rita Bovolenta (University of Ferrara, Italy) for help in some TEM analyses, and Sig. Brasola (La Rosa, Albinia, Italy) for the supply of experimental animals. This work was supported by grants from MIUR (ex 60%).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fausto, A.M., Picchietti, S., Taddei, A.R. et al. Formation of the egg envelope of a teleost, Dicentrarchus labrax (L.): immunochemical and cytochemical detection of multiple components. Anat Embryol 208, 43–53 (2004). https://doi.org/10.1007/s00429-003-0372-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-003-0372-z