Abstract
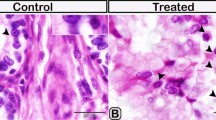
A recent observation concerning the phallus of the tinamou Nothura maculosa was the presence of cells resembling plasma cells within the epithelium of the fixed tubular portion. Owing to this unusual location of plasma cells, we studied the phalli of the tinamous N. maculosa and Rhynchotus rufescens to confirm the occurrence of intraepithelial plasma cells and to evaluate the seasonal variation in these cells. Abundant plasma cells were found within the epithelium of the fixed tubular portion of the phallus but not in the evertible portion. Migration of plasma cells from the adjacent connective tissue through the basement membrane and between the epithelial cells was frequent. Some plasma cells exhibited a rough endoplasmic reticulum with variable cisternal distension, containing fine, slightly electron-dense, granular material suggestive of immunoglobulin accumulation. An expressive increase of more than 800% in the number of intraepithelial plasma cells was found during the breeding season compared to the non-breeding season. By establishing the occurrence of intraepithelial plasma cells in the phallus and their seasonal variation, the results contribute to a better understanding of the role of these cells in the mucosal immune system of the reproductive tract of male birds.




Similar content being viewed by others
References
Ahlqvist J, Anderson L (1972) Methyl green-pyronin staining: effects of fixation; use in routine pathology. Stain Technol 47:17–22
Bang BG, Bang FB (1968) Localized lymphoid tissues and plasma cells in paraocular and paranasal organ systems in chickens. Am J Pathol 53:735–751
Brink PR, Walcott B, Roemer E, Grine E, Pastor M, Christ GJ, Cameron RH (1994) Cholinergic modulation of immunoglobulin secretion from avian plasma cells: the role of calcium. J Neuroimmunol 51:113–121
Briskie JV, Montgomerie R (1997) Sexual selection and the intromittent organ of birds. J Avian Biol 28:73–86
Casamayor-Pallejà M, Mondiére P, Amara A, Bella C, Dieu-Nosjean MC, Caux C, Defrance T (2001) Expression of macrophage inflammatory protein-3α, stromal cell-derived factor-1, and B-cell-attracting chemokine-1 identifies the tonsil crypt as an attractive site for B cells. Blood 97:3992–3994
Chin KN, Wong WC (1977) Some ultrastructural observations on the intestinal mucosa of the toad (Bufo melanostictus). J Anat 123:331–339
Cowden RR, Dyer RF (1971) Lymphopoietic tissue and plasma cells in amphibians. Am Zool 11:183–192
Cyster JC (2003) Homing of antibody secreting cells. Immunol Rev 194:48–60
Del Hoyo J, Elliot A, Saragatal J (1992) Class Aves, order Tinamiformes, family Tinamidae. In: Del Hoyo J, Elliot A, Saragatal J (eds) Handbook of the birds of the world (eds). Lynx Edidions, Barcelona, pp 112–138
Fange R, Silverin B (1985) Variation of lymphoid activity in the spleen of a migratory bird, the pied flycatcher (Ficedula hypoleuca; Aves, Passeriformes). J Morphol 184:33–40
Folstad I, Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. Am Naturalist 139:603–622
Grossman CJ (1985) Interactions between the gonadal steroids and the immune system. Science 227:257–261
Guzsal E (1974) Erection apparatus of the copulatory organ of ganders and drakes. Acta Vet Acad Sci Hung 24:361–373
Hess RA, Gist DH, Bunick D, Lubahn DB, Farrell A, Bahr J, Cooke PS, Greene GL (1997) Estrogen receptor (alpha and beta) expression in the excurrent ducts of the adult male rat reproductive tract. J Androl 18:602–611
Hess RA, Zhou Q, Nie R, Oliveira C, Cho H, Nakaia M, Carnes K (2001) Estrogens and epididymal function. Reprod Fertil Dev 13:273–283
John JL (1994) The avian spleen: a neglected organ. Q Rev Biol 69:327–351
Johnson GD, Ling NR, Nathan PD, Hardie DL (1986) Use of monoclonal antibodies reactive with secretory epithelial cells for the immunocytochemical identification of plasma cells. Immunol Lett 12:295–300
Khan MZ, Hashimoto Y, Iwami Y, Iwanaga T (1997) Postnatal development of B lymphocytes and immunoglobulin-containing plasma cells in the chicken oviduct: studies on cellular distribution and influence of sex hormones. Vet Immunol Immunopathol 56:329–338
Komarek V, Marvan F (1969) Contribution to the microscopic anatomy of the copulation organ of the Anatidae. Anat Anz 124:467–476
Kunkel E, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC (2003) CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA-Ab secreting cells. J Clin Invest 111:1001–1010
Kwon S, Hess RA, Bunick D, Kirby JD, Bahr JM (1997) Estrogen receptors are present in the epididymis of the rooster. J Androl 18:378–384
Lake PE (1981) Male genital organs. In: King AS, McLelland J (eds) Form and function in birds. Academic Press, London, pp 1–61
Lefevre ME, Reincke U, Arbas R, Gennaro JF (1973) Lymphoid cells in the turtle bladder. Anat Rec 176:111–120
Leitner G, Landsman T, Blum O, Zaltsmann N, Heller ED (1996) Effects of gonadal steroids and their antagonists on the humoral immune response of immune-selected broiler chicks. Poult Sci 75:1373–1382
Linder JE, Hopps RM, Johnson NW (1977) Methyl green-pyronin with hematoxylin and orange G for the identification of inflammatory cells in tissue sections. Stain Technol 52:233–236
Muir WI, Bryden WL, Husband AJ (2000) Investigation of the site of precursors for IgA-producing cells in the chicken intestine. Immunol Cell Biol 78:294–296
Nickerson SC, Pankey JW, Boddie NT (1984) Distribution, location, and ultrastructure of plasma cells in the uninfected, lactating bovine mammary gland. J Dairy Res 51:209–217
Nishiyama H (1955) Studies on the accessory reproductive organs in the cock. J Fac Agr Kyushu 10:277–306
Oláh I, Scott TR, Gallego M, Kendall C, Glick B (1992) Plasma cells expressing immunoglobulins M and A but not immunoglobulin G develop an intimate relationship with central canal epithelium in the harderian gland of the chicken. Poult Sci 71:664–676
Oláh I, Kupper A, Kittner Z (1996) The lymphoid substance of the chicken’s Harderian gland is organized in two histologically distinct compartments. Microsc Res Tech 34:166–176
Oliveira CA, Mahecha GA (2000) Morphology of the copulatory apparatus of the spotted tinamou Nothura maculosa (Aves: Tinamiformes). Anat Anz 182:161–169
Opstad AM (1959) A methyl green pyronin stain for plasma cells in tissues. Stain Technol 34:293
Raj GD, Jones RC (1996) Local antibody production in the oviduct and gut of hens infected with a variant strain of infectious bronchitis virus. Vet Immunol Immunopathol 53:147–161
Rideout BA, Lowensteine LJ, Hutson CA, Moore PF, Pedersen NC (1992) Characterization of morphologic changes and lymphocyte subset distribution in lymph nodes from cats with naturally acquired feline immunodeficiency virus infection. Vet Pathol 29:391–399
Russell LD, Ren HP, Sinha Hikim I, Schulze W, Sinha Hikim AP (1990) A comparative study in twelve mammalian species of volume densities, volumes, and numerical densities of selected testis components, emphasizing those related to the Sertoli cell. Am J Anat 188:21–30
Schramm U (1980) Lymphoid cells in the Harderian gland of birds. An electron microscopical study. Cell Tissue Res 205:85–94
Slifka MK, Ahmed R (1998) Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol 10:252–258
Solas MT, Zapata A (1980) Gut-associated lymphoid tissue (GALT) in reptiles: intraepithelial cells. Dev Comp Immunol 4:87–97
Tomonaga S, Zhang H, Kobayashi K, Fujii R, Teshima K (1992) Plasma cells in the spleen of the Aleutian skate, Bathyraja aleutica. Arch Histol Cytol 55:287–294
Veloso FT, Saleiro JV (1981) Heterogeneity of plasma cells in Crohn’s disease. J Submicrosc Cytol 13:57–62
von Rautenfeld DB, Budras KD, Gassmann R (1976) A morphological study of antibody transport in the transparent fluid flowing from the lymph-folds of the copulatory organ into the cloacal lumen of the cock (Gallus domesticus). Z Mikrosk Anat Forsch 90:989–1008
Weibel ER, Staubli W, Gnagi HR, Hess FA (1969) Correlated morphometric and biochemical studies on the liver cell. I. Morphometric model, stereologic methods, and normal morphometric data for rat liver. J Cell Biol 42:68–91
Zheng WM, Yoshimura Y, Tamura T (1997) Effects of sexual maturation and gonadal steroids on the localization of IgG-, IgM- and IgA-positive cells in the chicken oviduct. J Reprod Fertil 111:277–84
Zheng WM, Izaki J, Furusawa S, Yoshimura Y (2000) Localization of immunoglobulin G gamma-chain mRNA-expressing cells in the oviduct of laying and diethylstilbestrol-treated immature hens. Gen Comp Endocrinol 120:345–352
Zicca A, Cadoni A, Leprini A, Millo R, Lydyard PM, Grossi CE (1982) Immunofluorescent and ultrastructural analysis of plasma cell degeneration in the chicken Harder’s gland. Dev Comp Immunol 6:131–139
Zuk M (1990) Reproductive strategies and disease susceptibility: an evolutionary viewpoint. Parasitol Today 6:231–233
Acknowledgements
This work was supported by the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq-UFMG). The authors wish to thank Dr. Greg Kitten for revision of the manuscript and valuable suggestions and the Center of Electron Microscopy (CEMEL) of the UFMG for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oliveira, C.A., Geraldo, I., Poblete, P.C.P. et al. Intraepithelial plasma cells in the avian copulatory organ of two tinamou species: quantitative variation during the breeding season. Anat Embryol 207, 409–416 (2003). https://doi.org/10.1007/s00429-003-0358-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-003-0358-x