Abstract
Aims
Cutaneous syncytial myoepithelioma (CSM) is a rare myoepithelioma variant of skin, characterized by intradermal syncytial growth of spindle cells with a distinct immunophenotype of EMA and S100 positivity and infrequent keratin expression. While CSM was first described as a cutaneous tumor, singular non-cutaneous cases have since been reported in bone. We aimed to investigate the clinicopathological features of this variant across all anatomic sites through a large multi-institutional study.
Methods and results
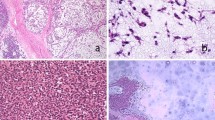
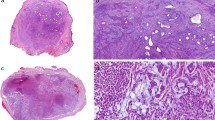
We complied a total of 24 myoepitheliomas with syncytial growth from our files. The tumors occurred in 12 male and 12 female patients (M:F = 1:1), with a median age of 31 years (range, 9–69 years). While the majority of tumors (75%, n = 18) occurred in skin, a significant subset (25%, n = 6) arose in non-cutaneous sites, including bone (n = 3), bronchus/trachea (n = 2), and interosseous membrane of tibia/fibula (n = 1). Tumor size ranged from 0.4 to 5.9 cm. Clinical follow-up (7 patients; range 14–202 months; median 56.5 months) showed a single local recurrence 8 years after incomplete skin excision but no metastases; all patients were alive at the time of last follow-up without evidence of disease. Histologically, all tumors were pink at low-power and characterized by a syncytial growth of bland ovoid, spindled, or histiocytoid cells with eosinophilic cytoplasm and prominent perivascular lymphoplasmacytic inflammation. One-third displayed adipocytic metaplasia (8/24). Rare cytologic atypia was seen but was not associated with increased mitotic activity. All tumors expressed S100, SMA, and/or EMA. Keratin expression was absent in most cases. Molecular analysis was performed in 16 cases, all showing EWSR1-rearrangments. In total, 15/15 (100%) harbored an EWSR1::PBX3 fusion, whereas 1 case EWSR1 FISH was the only molecular study performed.
Conclusion
Syncytial myoepithelioma is a rare but recognizable morphologic variant of myoepithelioma which may have a predilection for skin but also occurs in diverse non-cutaneous sites. Our series provides evidence supporting a reappraisal of the term “cutaneous syncytial myoepithelioma,” as 25% of patients in our series presented with non-cutaneous tumors; thus, we propose the term “syncytial myoepithelioma” to aid pathologist recognition and avoidance of potentially confusing terminology when referring to non-cutaneous examples. The behavior of syncytial myoepithelioma, whether it arises in cutaneous or non-cutaneous sites, is indolent and perhaps benign with a small capacity for local recurrence.




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available upon request from the corresponding author.
References
Jo VY, Antonescu CR, Zhang L, Dal Cin P, Hornick JL, Fletcher CDM (2013) Cutaneous syncytial myoepithelioma: clinicopathologic characterization in a series of 38 cases. Am J Surg Pathol 37:710–718
Jo VY, Antonescu CR, Dickson BC, Swanson D, Zhang L, Fletcher CDM et al (2019) Cutaneous syncytial myoepithelioma is characterized by recurrent EWSR1-PBX3 fusions. Am J Surg Pathol 43:1349–1354
Lee JH, Huang HY, Lan J, Hwang CC, Liu CY (2015) Cutaneous syncytial myoepithelioma: a case report with emphasis on the differential diagnosis of problematic dermal tumors. Oncol Lett 9:2275–2277
Pizzi M, Facchin F, Kohlscheen E, Sartore L, Salmaso R, Bassetto F (2016) Cutaneous syncytial myoepithelioma: clinico-pathological features and differential diagnosis. Pathol Res Pract 212:954–956
Alomari AK, Brown N, Andea AA, Betz BL, Patel RM (2017) Cutaneous syncytial myoepithelioma: a recently described neoplasm which may mimic nevoid melanoma and epithelioid sarcoma. J Cutan Pathol 44:892–897
Wales C, Diamond S, Hinds B (2020) Cutaneous syncytial myoepithelioma: a nondescript skin tumor with serious diagnostic pitfalls. Int J Surg Pathol 28:63–67
Shimada K, Ansai O, Katsumi T, Deguchi T, Hayashi R, Yuki A et al (2022) A case of cutaneous syncytial myoepithelioma with extensive adipocytic metaplasia: usefulness of EWSR1-PBX3 gene fusion analysis. J Cutan Pathol 49:412–417
MacKinnon WF, Carter MD, Bridge JA, Tremaine RD, Walsh NMG (2019) EWSR1-PBX3 gene fusion in cutaneous syncytial myoepithelioma. J Cutan Pathol 46:421–424
Skalova A, Vanecek T, Martinek P, Weinreb I, Stevens TM, Simpson RHW et al (2018) Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel ETV6-RET translocation: report of 10 Cases. Am J Surg Pathol 42:234–246
Hornick JL, Fletcher CDM (2004) Cutaneous myoepithelioma: a clinicopathologic and immunohistochemical study of 14 cases. Hum Pathol 35:14–24
Agaram NP, Chen HW, Zhang L, Sung YS, Panicek D, Healey JH et al (2015) EWSR1-PBX3: a novel gene fusion in myoepithelial tumors. Genes, Chromosom Cancer 54:63–71
Suurmeijer AJH, Dickson BC, Swanson D, Zhang L, Sung YS, Fletcher CD, Antonescu CR (2020) A morphologic and molecular reappraisal of myoepithelial tumors of soft tissue, bone, and viscera with EWSR1 and FUS gene rearrangements. Genes Chromosom Cancer 59(6):348–356
Schnabel CA, Selleri L, Jacobs Y, Warnke R, Cleary ML (2001) Expression of Pbx1b during mammalian organogenesis. Mech Dev 100:131–135
Laurent A, Bihan R, Omilli F, Deschamps S, Pellerin I (2008) PBX proteins: much more than Hox cofactors. Int J Dev Biol 52(1):9–20
Han HB, Gu J, Zuo HJ, Chen ZG, Zhao W, Li M et al (2012) Let-7c functions as a metastasis suppressor by targeting MMP11 and PBX3 in colorectal cancer. J Pathol 226(3):544–555
Ramberg H, Alshbib A, Berge V, Svindland A, Taskén KA (2011) Regulation of PBX3 expression by androgen and Let-7d in prostate cancer. Mol Cancer 10:50
Li H, Sun G, Liu C, Wang J, Jing R, Wang J et al (2017) PBX3 is associated with proliferation and poor prognosis in patients with cervical cancer. Onco Targets Ther 10:5685–5694
Li Y, Sun Z, Zhu Z, Zhang J, Sun X, Xu H (2014) PBX3 is overexpressed in gastric cancer and regulates cell proliferation. Tumour Biol 35(5):4363–4368
Li Z, Zhang Z, Li Y, Arnovitz S, Chen P, Huang H et al (2013) PBX3 is an important cofactor of HOXA9 in leukemogenesis. Blood 121:1422–1431
Panagopoulos I, Gorunova L, Bjerkehagen B, Heim S (2015) Fusion of the genes EWSR1 and PBX3 in retroperitoneal leiomyoma with t(9;22)(q33;q12). PLoS One 10:e012428
Panagopoulos I, Gorunova L, Brunetti M, Agostini A, Andersen HK, Lobmaier I et al (2017) Genetic heterogeneity in leiomyomas of deep soft tissue. Oncotarget 8:48769–48781
Haglund C, Zemmler M, Tsagkozis P, Haglund de Flon F (2023) An intraosseous myoepithelial carcinoma with a EWSR1::PBX3 fusion. Genes Chromosom Cancer 2. https://doi.org/10.1002/gcc.2314
Hornick JL, Dal Cin P, Fletcher CDM (2009) Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 33:542–550
Jo VY, Fletcher CDM (2017) SMARCB1/INI1 Loss in epithelioid schwannoma: a clinicopathologic and immunohistochemical study of 65 cases. Am J Surg Pathol 41:1013–1022
Doyle LA, Fletcher CDM (2011) EMA positivity in epithelioid fibrous histiocytoma: a potential diagnostic pitfall. J Cutan Pathol 38:697–703
Doyle LA, Mariño-Enriquez A, Fletcher CDM, Hornick JL (2015) ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol 28:904–912
Agaram NP, Zhang L, Sung YS, Singer S, Stevens T, Prieto-Granada CN, Bishop JA, Wood BA, Swanson D, Dickson BC, Antonescu CR (2019) GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions. Mod Pathol 32:1617–1626
Antonescu CR, Agaram NP, Sung YS, Zhang L, Swanson D, Dickson BC (2018) A distinct malignant epithelioid neoplasm with GLI1 gene rearrangements, frequent S100 protein expression, and metastatic potential: expanding the spectrum of pathologic entities with ACTB/MALAT1/PTCH1-GLI1 fusions. Am J Surg Pathol 42:553–560
Liu J, Mao R, Lao IW, Yu L, Bai Q, Zhou X, Wang J (2022) GLI1-altered mesenchymal tumor: a clinicopathological and molecular analysis of ten additional cases of an emerging entity. Virchows Arch 480:1087–1099
Cloutier JM, Charville GW, Mertens F, Sukov W, Fritchie K, Perry KD, Edgar M, Rowsey RA, Folpe AL (2021) “Inflammatory leiomyosarcoma” and “histiocyte-rich rhabdomyoblastic tumor”: a clinicopathological, immunohistochemical and genetic study of 13 cases, with a proposal for reclassification as “inflammatory rhabdomyoblastic tumor”. Mod Pathol 34:758–769
Hornick JL, Fletcher CD (2005) Soft tissue perineurioma: clinicopathologic analysis of 81 cases including those with atypical histologic features. Am J Surg Pathol 29:845–858
Mariño-Enríquez A, Fletcher CD (2012) Angiofibroma of soft tissue: clinicopathologic characterization of a distinctive benign fibrovascular neoplasm in a series of 37 cases. Am J Surg Pathol 36(4):500–508
Yamashita K, Baba S, Togashi Y, Dobashi A, Ae K, Matsumoto S, Tanaka M, Nakamura T, Takeuchi K (2023) Clinicopathologic and genetic characterization of angiofibroma of soft tissue: a study of 12 cases including two cases with AHRR::NCOA3 gene fusion. Histopathology 83:57–66
Agaimy A, Perret R, Demicco EG, Gross J, Liu YJ, Azmani R, Engelmann C, Schubart C, Seppet J, Stoehr R, Le Loarer F, Dickson BC (2023) GAB1::ABL1 fusions define a distinctive soft tissue neoplasm, with variable perineurial differentiation, and a predilection for children and young adults. Genes Chromosom Cancer 62:449–459
Rekhi B, Antonescu CR, Chen G (2022) Angiomatoid fibrous histiocytoma. In: WHO classification of tumors of soft tissue and bone, vol 3, 5th edn. International Agency for Research on Cancer, Lyons, pp 271–273
Cheah AL, Zou Y, Lanigan C, Billings SD, Rubin BP, Hornick JL, Goldblum JR (2019) ALK Expression in angiomatoid fibrous histiocytoma: a potential diagnostic pitfall. Am J Surg Pathol 43:93–101
Baumhoer D, Rogozhin D (2022) Non-ossifying fibroma. In: WHO classification of tumors of soft tissue and bone, 5th edn. International Agency for Research on Cancer, Lyons, pp 447–448
Diaz-Perez JA, Nielsen GP, Antonescu C, Taylor MS, Lozano-Calderon SA, Rosenberg AE (2019) EWSR1/FUS-NFATc2 rearranged round cell sarcoma: clinicopathological series of 4 cases and literature review. Hum Pathol 90:45–53
Sigel JE, Smith TA, Reith JD, Goldblum JR (2001) Immunohistochemical analysis of anaplastic lymphoma kinase expression in deep soft tissue calcifying fibrous pseudotumor: evidence of a late sclerosing stage of inflammatory myofibroblastic tumor? Ann Diagn Pathol 5:10–14
Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM et al (2001) Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol 25:1364–1371
Cools J, Wlodarska I, Somers R, Mentens N, Pedeutour F, Maes B et al (2002) Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosom Cancer 34:354–362
Coffin CM, Hornick JL, Fletcher CDM (2007) Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31:509–520
Thway K, Nicholson AG, Lawson K, Gonzalez D, Rice A, Balzer B, Swansbury J, Min T, Thompson L, Adu-Poku K, Campbell A, Fisher C (2011) Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion: a new tumor entity. Am J Surg Pathol 35:1722–1732
Enzinger FM, Harvey DA (1975) Spindle cell lipoma. Cancer 36:1852–1859
Mariño-Enriquez A, Nascimento AF, Ligon AH, Liang C, Fletcher CD (2017) Atypical spindle cell lipomatous tumor: clinicopathologic characterization of 232 cases demonstrating a morphologic spectrum. Am J Surg Pathol 41:234–244
Gross JM, Perret R, Coindre JM, Le Loarer F, Michal M, Michal M, Miettinen M, McCabe CE, Nair AA, Swanson AA, Thangaiah JJ, Torres-Mora J, Bonadio A, Voltaggio L, Epstein JI, Gupta S, Folpe AL, Schoolmeester JK (2023) Lipoblastoma-like tumor and fibrosarcoma-like lipomatous neoplasm represent the same entity: a clinicopathologic and molecular genetic study of 23 cases occurring in both men and women at diverse locations. Mod Pathol 36:100246. https://doi.org/10.1016/j.modpat.2023.100246
Anderson WJ, Mariño-Enríquez A, Trpkov K, Hornick JL, Nucci MR, Dickson BC, Fletcher CDM (2023) Expanding the clinicopathologic and molecular spectrum of lipoblastoma-like tumor in a series of 28 cases. Mod Pathol 100252. https://doi.org/10.1016/j.modpat.2023.100252
Clay MR, Martinez AP, Weiss SW, Edgar MA (2015) MDM2 amplification in problematic lipomatous tumors: analysis of FISH testing criteria. Am J Surg Pathol 39:1433–1439
Acknowledgements
The authors thank Norman Barker, MS, MA, RBP for his assistance with preparing figures for this article.
Author information
Authors and Affiliations
Contributions
SW reviewed all cases, analyzed data, and wrote the first draft of the manuscript. SGG, AB, JTM, YSZ, MM, and GWC contributed to data collection. JMG designed the study, reviewed all cases, and reviewed and edited the manuscript. All authors edited the manuscript and read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The authors declare the compliance of ethical standards in research. This study was approved by the Johns Hopkins Institutional Review Board (IRB00379801, 3/21/2023).
Consent for publication
The data are not publicly available due to privacy or ethical restrictions.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wangsiricharoen, S., Gjeorgjievski, S.G., Bahrami, A. et al. Non-cutaneous syncytial myoepitheliomas are identical to cutaneous counterparts: a clinicopathologic study of 24 tumors occurring at diverse locations. Virchows Arch 483, 665–675 (2023). https://doi.org/10.1007/s00428-023-03609-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03609-3