Abstract
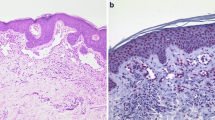
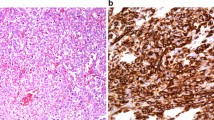
Angiosarcoma is a malignant vascular endothelial neoplasm with various histological patterns. Despite its highly malignant potential, histological prognostic prediction has not been adopted for angiosarcoma. This study aimed to establish a method of predicting the prognosis of primary angiosarcoma. Formalin-fixed, paraffin-embedded samples from 104 primary angiosarcomas were prepared. All the cases were reviewed based on histological examinations with H&E staining. Because the French Fédération Nationale des Centres de Lutte Contre Le Cancer system (FNCLCC) is not adopted for angiosarcoma, we experimentally established a modified version of FNCLCC. Immunohistochemical staining for ERG, CD31, CD34, D2-40, HHV-8, p16, C-MYC, and p53 was performed. Fluorescence in situ hybridization (FISH) was performed for 31 cases to assay c-MYC gene amplification. Multivariate analysis revealed that age (> 70 years old) (p = 0.0011), non-cutaneous angiosarcoma (p = 0.0265), metastasis on diagnosis (p < 0.0001), size ≥ 5 cm (p = 0.0388), no taxane chemotherapy (p = 0.0388), strong nuclear atypia (p = 0.0087), and the presence of luminal structure in ≥ 50% of the tumor volume (p = 0.0009) were independent poor prognostic factors. Among angiosarcomas with luminal formation, mFNCLCC scores were significantly correlated with a poorer prognosis. The overexpression of p16 was associated with less luminal formation (p = 0.0192). Immunohistochemical analysis of C-MYC showed a moderate level of concordance with FISH (Kappa value = 0.45). This study suggested that luminal formation and nuclear atypia may be poor histological prognostic factors of angiosarcoma and that mFNCLCC would be useful for predicting the prognosis of angiosarcoma with luminal formation.



Similar content being viewed by others
References
Billings SD, Brenn T, Hornick JL (2018) Cutaneous angiosarcoma. In: Elder DE, Massi D, Scolyer RA, Willemze R et al (eds) World Health Organization Classification of Skin Tumours. IARC Press, Lyon, France, pp 335–336
Thomas K, Billing SD. Angiosarcoma (2020) In: World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone, 5th ed. Lyon, France: IARC Press, pp 176–178
Young RJ, Brown NJ, Reed MW et al (2010) Angiosarcoma. Lancet Oncol 11:983–991. https://doi.org/10.1016/S1470-2045(10)70023-1
Deyrup AT, McKenney JK, Tighiouart M et al (2008) Sporadic Cutaneous Angiosarcomas: A Proposal for Risk Stratification Based on 69 Cases. Am J Surg Pathol 32:72–77. https://doi.org/10.1097/PAS.0b013e3180f633a3
Dettenborn T, Wermker K, Schulze HJ et al (2014) Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg 42:1623–1628. https://doi.org/10.1016/j.jcms.2014.05.002
Pollock RE, Maki RG (2017) Introduction of soft tissue sarcoma. In: Amin MB, Edge SB, Greene FL, et al. eds. AJCC Cancer Staging Manual, 8th ed. Chicago, U.S.A.: SPRINGER-VERLAG, pp 489–497
Naka N, Ohsawa M, Tomita Y, Kanno H, Uchida A, Myoui A, Aozasa K (1996) Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. J Surg Oncol 61:170–176. https://doi.org/10.1002/(SICI)1096-9098(199603)61:3<170::AID-JSO2>3.0.CO;2-8
Sinnamon AJ, Neuwirth MG, McMillan MT et al (2016) A prognostic model for resectable soft tissue and cutaneous angiosarcoma. J Surg Oncol 114:557–563. https://doi.org/10.1002/jso.24352
Shon W, Jenkins SM, Ross DT et al (2011) Angiosarcoma: a study of 98 cases with immunohistochemical evaluation of TLE3, a recently described marker of potential taxane responsiveness. J Cutan Pathol 38:961–966. https://doi.org/10.1111/j.1600-0560.2011.01790.x
Buehler D, Rush P, Hasenstein JR (2013) Expression of Angiopoietin-TIE System Components in Angiosarcoma. Mod Pathol 26:1032–1040. https://doi.org/10.1038/modpathol.2013.43
Motaparthi K, Lauer SR, Patel RM et al (2020) MYC gene amplification by fluorescence in situ hybridization and MYC protein expression by immunohistochemistry in the diagnosis of cutaneous angiosarcoma: Systematic review and appropriate use criteria. J Cutan Pathol 1–9. https://doi.org/10.1111/cup.13912
Jiromaru R, Yamamoto H, Yasumatsu R et al (2020) HPV-related Sinonasal Carcinoma: Clinicopathologic Features, Diagnostic Utility of p16 and Rb Immunohistochemistry, and EGFR Copy Number Alteration. Am J Surg Pathol 44:305–315. https://doi.org/10.1097/PAS.0000000000001410
Italiano A, Chen CL, Thomas R et al (2012) Alterations of the p53 and PIK3CA/AKT/mTOR pathways in angiosarcomas: a pattern distinct from other sarcomas with complex genomics. Cancer 118:5878–5887. https://doi.org/10.1002/cncr.27614
Ginter PS, Mosquera JM, MacDonald TY et al (2014) Diagnostic utility of MYC amplification and anti-MYC immunohistochemistry in atypical vascular lesions, primary or radiation-induced mammary angiosarcomas, and primary angiosarcomas of other sites. Hum Pathol 45:709–716. https://doi.org/10.1016/j.humpath.2013.11.002
Brierly JD, Gospodarowicz MK, Wittekind C (2017) TNM classification of malignant tumors. Wiley-Blackwell, pp 124–126
Murali R, Chandramohan R, Möller I et al (2015) Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway. Oncotarget 6:36041–36052. https://doi.org/10.18632/oncotarget.5936
Shimozono N, Jinnin M, Masuzawa M et al (2015) NUP160–SLC43A3 is a novel recurrent fusion oncogene in angiosarcoma. Cancer Res 75:4458–44565. https://doi.org/10.1158/0008-5472.CAN-15-0418
Painter CA, Jain E, Tomson BN et al (2020) The Angiosarcoma Project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat Med 26:181–187. https://doi.org/10.1038/s41591-019-0749-z
Giatromanolaki A, Kouroupi M, Balaska K, Koukourakis MI (2020) Immunohistochemical detection of senescence markers in human sarcomas. Pathol Res Pract 216(2):152800. https://doi.org/10.1016/j.prp.2019.152800
Baruah P, Lee M, Wilson PO et al (2015) Impact of p16 status on pro- and anti-angiogenesis factors in head and neck cancers. Br J Cancer 113(4):653–659. https://doi.org/10.1038/bjc.2015.251
Zhang J, Lu A, Li L, Yue J, Lu Y (2010) p16 Modulates VEGF expression via its interaction with HIF-1alpha in breast cancer cells. Cancer Invest 28(6):588–597. https://doi.org/10.3109/07357900903286941
Eelen G, Treps L, Li X, Carmeliet P (2020) Basic and Therapeutic Aspects of Angiogenesis Updated. Circ Res 127(2):310–329. https://doi.org/10.1161/CIRCRESAHA.120.316851
Shon W, Sukov WR, Jenkins SM, Folpe AL (2014) MYC amplification and overexpression in primary cutaneous angiosarcoma: a fluorescence in-situ hybridization and immunohistochemical study. Mod Pathol 27:509–515. https://doi.org/10.1038/modpathol.2013.163
Udager AM, Ishikawa MK, Lucas DR et al (2016) MYC immunohistochemistry in angiosarcoma and atypical vascular lesions: practical considerations based on a single institutional experience. Pathology 48:697–704. https://doi.org/10.1016/j.pathol.2016.08.007
Machado I, Giner F, Lavernia J et al (2021) Angiosarcomas: histology, immunohistochemistry and molecular insights with implications for differential diagnosis. Histol Histopathol 36:3–18. https://doi.org/10.14670/HH-18-246
Donnell RM, Rosen PP, Lieberman PH et al (1981) Angiosarcoma and other vascular tumors of the breast. Am J Surg Pathol 5:629–642
Antonescu C (2014) Malignant vascular tumors–an update. Mod Pathol 27:S30-38. https://doi.org/10.1038/modpathol.2013.176
Kuba MG, Dermawan JK, Xu B et al (2023) Histopathologic Grading Is of Prognostic Significance in Primary Angiosarcoma of Breast: Proposal of a Simplified 2-tier Grading System. Am J Surg Pathol 47(3):303–317. https://doi.org/10.1097/PAS.0000000000001998
Acknowledgements
The authors thank Masayuki Hirose and Junji Kishimoto (Center for Clinical and Translational Research at Kyushu University Hospital) for reviewing the statistical methods of this study.
This work was supported by JSPS KAKENHI Grant Number 19H03444.
Author information
Authors and Affiliations
Contributions
T. Ichiki performed the research and wrote the paper. Y. Yamada and T. Ito contributed to the research design and slide review. Y. Nakashima, M. Nakamura, T. Yoshizumi, A. Shiose, and K. Akashi contributed to the acquisition of the samples. T. Nakahara and Y. Oda designed the research and gave final approval of the manuscript. All authors critically reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Ethical
This study was conducted in accordance with the principles embodied in the Declaration of Helsinki. This study was also approved by the Ethics Committee of Kyushu University (Nos. 29-625, 29-429, 2020-361, 2021-165).
Conflict of interest
The authors declare that we have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ichiki, T., Yamada, Y., Ito, T. et al. Histological and immunohistochemical prognostic factors of primary angiosarcoma. Virchows Arch 483, 59–69 (2023). https://doi.org/10.1007/s00428-023-03572-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03572-z