Abstract
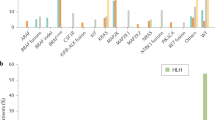
Diagnosis of histiocytosis can be difficult and one of the biggest challenges is to distinguish between reactive and neoplastic histiocytes on histology alone. Recently, OCT2 nuclear expression was reported in Rosai-Dorfman disease (RDD). Our purpose was to expand the testing of OCT2 on a broader variety of sporadic or H syndrome-related histiocytoses. Cases of histiocytoses were retrieved from the files of Ambroise Paré Pathology Department. All slides and molecular analyses were reviewed, and staining was completed with immunohistochemistry for OCT2. A total of 156 samples from different localizations were tested. Among sporadic cases, 52 patients had RDD, and 10 patients had mixed histiocytosis combining RDD with Erdheim Chester disease (ECD, n = 8), Langerhans cell histiocytosis (LCH, n = 2) or juvenile xanthogranuloma (JXG, n = 1). All these patients were positive for OCT2 in RDD characteristic histiocytes. Twenty-three patients had ECD and all but two (91% − 21/23) were negative for OCT2. By contrast, OCT2 was positive in 11/27 (41%) LCH and 6/16 (38%) JXG. Among the 10 samples of H syndrome-associated histiocytosis, 3 had typical RDD histology, 6 had unclassified histiocytosis, and one had mixed RDD-LCH; all were positive for OCT2. On 16 samples of granulomatous lymphadenitis, OCT2 was negative in epithelioid histiocytes. Our study shows that OCT2 has a sensitivity of 100% for RDD cases and mixed histiocytoses with an RDD component. It is negative in 92% of ECD but expressed in at least 38% of LCH, JXG, and C group histiocytoses. Finally, OCT2 is positive in all H syndrome-related histiocytoses, independent of their histology.


Similar content being viewed by others
References
Emile JF, Cohen-Aubart F, Collin M et al (2021) Histiocytosis. Lancet 398(10295):157–170
Durham BH, Lopez Rodrigo E, Picarsic J et al (2019) Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med 25(12):1839–1842
Molho-Pessach V, Ramot Y, Camille F et al (2014) H syndrome: the first 79 patients. J Am Acad Dermatol 70(1):80–88
Yin L, Xu J, Li M et al (2016) Oct2 and Bob1 are sensitive and specific markers in lineage determination of B cell lymphomas with no expression of conventional B cell markers. Histopathology 69(5):775–783
Liu YZ, Dawson SJ, Latchman DS (1996) Alternative splicing of the Brn-3a and Brn-3b transcription factor RNAs is regulated in neuronal cells. J Mol Neurosci 7(1):77–85
Ravindran A, Goyal G, Go RS et al (2021) Rosai-Dorfman disease displays a unique monocyte-macrophage phenotype characterized by expression of OCT2. Am J Surg Pathol 45(1):35–44
Kiruthiga KG, Younes S, Natkunam Y (2022) Strong coexpression of transcription factors PU.1 and Oct-2 in Rosai-Dorfman disease. Am J Clin Pathol 158(6):672–677
Kemps PG, Picarsic J, Durham BH et al (2022) ALK-positive histiocytosis: a new clinicopathologic spectrum highlighting neurologic involvement and responses to ALK inhibition. Blood 139(2):256–280
Wang E, Hutchinson CB, Huang Q et al (2010) Histiocytic sarcoma arising in indolent small B-cell lymphoma: report of two cases with molecular/genetic evidence suggestive of a “transdifferentiation” during the clonal evolution. Leuk Lymphoma 51(5):802–812
Emile JF, Abla O, Fraitag S et al (2016) Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 127(22):2672–2681
Morgan NV, Morris MR, Cangul H et al (2010) Mutations in SLC29A3, encoding an equilibrative nucleoside transporter ENT3, cause a familial histiocytosis syndrome (Faisalabad histiocytosis) and familial Rosai-Dorfman disease. PLoS Genet 6(2):e1000833
Emile JF, Diamond EL, Hélias-Rodzewicz Z et al (2014) Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood 124(19):3016–3019
Melloul S, Hélias-Rodzewicz Z, Cohen-Aubart F et al (2019) Highly sensitive methods are required to detect mutations in histiocytoses. Haematologica 104(3):e97–e99
Bolze A, Abhyankar A, Grant AV et al (2012) A mild form of SLC29A3 disorder: a frameshift deletion leads to the paradoxical translation of an otherwise noncoding mRNA splice variant. PLoS One 7(1):e29708
Chouk H, Ben Rejeb M, Boussofara L et al (2021) Phenotypic intrafamilial variability including H syndrome and Rosai-Dorfman disease associated with the same c.1088G > A mutation in the SLC29A3 gene. Hum Genomics 15:63
Doviner V, Maly A, Ne’eman Z et al (2010) H syndrome: recently defined genodermatosis with distinct histologic features. A morphological, histochemical, immunohistochemical, and ultrastructural study of 10 cases. Am J Dermatopathol 32(2):118–28
Razanamahery J, Diamond EL, Cohen-Aubart F et al (2020) Erdheim-Chester disease with concomitant Rosai-Dorfman like lesions: a distinct entity mainly driven by MAP2K1. Haematologica 105(1):e5–e8
Latchman DS (1996) The Oct-2 transcription factor. Int J Biochem Cell Biol 28(10):1081–1083
Lillycrop KA, Latchman DS (1992) Alternative splicing of the Oct-2 transcription factor RNA is differentially regulated in neuronal cells and B cells and results in protein isoforms with opposite effects on the activity of octamer/TAATGARAT-containing promoters. J Biol Chem 267(35):24960–24965
Lillycrop KA, Estridge JK, Latchman DS (1993) The octamer binding protein Oct-2 inhibits transactivation of the herpes simplex virus immediate-early genes by the virion protein Vmw65. Virology 196(2):888–891
Fraitag S, Emile JF (2022) Cutaneous histiocytoses in children. Histopathology 80(1):196–215
Funding
This article was supported by the Programme de Recherche Translationnelle sur le Cancer from the French National Cancer Institute [PRT-K19-143] and by unrestricted grants from the non-profit association AREP.
IAU received support from the European Society of Pathology for this work.
Author information
Authors and Affiliations
Contributions
IAU and JFE wrote de manuscript. JFE obtained funding. All co-authors contributed to collection and analysis of data, and correction of the manuscript. All co-authors accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The article is an original work. The study (NCT04437381) has been approved by the ethical committee CESREES #2814848bis. Patients signed informed consent for translational research. This work was presented in part during the 38th annual meeting of the Histiocyte Society (Stockholm, September 2022).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ungureanu, I.A., Cohen-Aubart, F., Héritier, S. et al. OCT2 expression in histiocytoses. Virchows Arch 483, 81–86 (2023). https://doi.org/10.1007/s00428-023-03508-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03508-7