Abstract
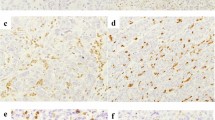
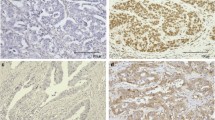
Survival benefits or symptom alleviation from immune checkpoint blockade therapy can be seen in microsatellite instability-high (MSI-H) cases. However, genetic heterogeneity within a specific subgroup of MSI-H tumors may be associated with poor response and prognosis. We investigated the molecular changes and microsatellite status of the cases with heterogeneous MMR protein staining by polymerase chain reaction (PCR) and next-generation sequencing (NGS). Data from 3723 patients with gastric cancer were retrospectively analyzed to determine the mismatch repair (MMR) status by performing immunohistochemical staining of four major MMR proteins (MLH1, PMS2, MSH2, and MSH6). When heterogeneous MMR protein staining result was positive, PCR and NGS were performed. Heterogeneous MMR protein staining was observed in 12 cases. In microsatellite stable (MSS) cases, TP53 mutation appeared to accompany heterogeneous staining (HS) of MLH1. However, TP53 variation was not observed with MSI-H occurrence. Cases showing heterogeneous MSH6 protein staining revealed MSH6 mutations. Some cases with the same MMR protein staining set had varying MSI results. In one case whose primary and metastatic foci presented MLH1-HS and PMS2-HS, the microsatellite status was classified as MSS and MSI-H, respectively. Moreover, HS was also found in multiple biopsies and surgical specimens. This study found a preliminary relationship between heterogeneously stained MSH6 or MLH1 proteins and their gene mutations, as well as between MSI-H/TP53 − and MSS/TP53 + tumors. The microsatellite status of patients with heterogeneous MMR protein staining is unpredictable. Given the heterogeneity of mismatch repair, microsatellite status should be assessed for all specimens if sufficient specimens can be obtained.


Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):359–386. https://doi.org/10.1002/ijc.29210
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132. https://doi.org/10.3322/caac.21338
Cancer Genome Atlas Research N (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513(7517):202–209. https://doi.org/10.1038/nature13480
Marcus L, Lemery SJ, Keegan P, Pazdur R (2019) FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res 25(13):3753–3758. https://doi.org/10.1158/1078-0432.CCR-18-4070
Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA et al (2017) Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 18(9):1182–1191. https://doi.org/10.1016/S1470-2045(17)30422-9
Lemery S, Keegan P, Pazdur R (2017) First FDA approval agnostic of cancer site - when a biomarker defines the indication. N Engl J Med 377(15):1409–1412. https://doi.org/10.1056/NEJMp1709968
Germano G, Lamba S, Rospo G, Barault L, Magri A, Maione F et al (2017) Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 552(7683):116–120. https://doi.org/10.1038/nature24673
Hu W, Yang Y, Ge W, Zheng S (2019) Deciphering molecular properties of hypermutated gastrointestinal cancer. J Cell Mol Med 23(1):370–379. https://doi.org/10.1111/jcmm.13941
Hu W, Yang Y, Qi L, Chen J, Ge W, Zheng S (2019) Subtyping of microsatellite instability-high colorectal cancer. Cell Commun Signal 17(1):79. https://doi.org/10.1186/s12964-019-0397-4
Yang Y, Shi Z, Bai R, Hu W (2021) Heterogeneity of MSI-H gastric cancer identifies a subtype with worse survival. J Med Genet 58(1):12–19. https://doi.org/10.1136/jmedgenet-2019-106609
Jiricny J (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7(5):335–346. https://doi.org/10.1038/nrm1907
Baretti M, Le DT (2018) DNA mismatch repair in cancer. Pharmacol Ther 189:45–62. https://doi.org/10.1016/j.pharmthera.2018.04.004
Kato A, Sato N, Sugawara T, Takahashi K, Kito M, Makino K et al (2016) Isolated loss of PMS2 immunohistochemical expression is frequently caused by heterogenous MLH1 promoter hypermethylation in Lynch syndrome screening for endometrial cancer patients. Am J Surg Pathol 40(6):770–776. https://doi.org/10.1097/PAS.0000000000000606
Pai RK, Plesec TP, Abdul-Karim FW, Yang B, Marquard J, Shadrach B et al (2015) Abrupt loss of MLH1 and PMS2 expression in endometrial carcinoma: molecular and morphologic analysis of 6 cases. Am J Surg Pathol 39(7):993–999. https://doi.org/10.1097/PAS.0000000000000415
Graham RP, Kerr SE, Butz ML, Thibodeau SN, Halling KC, Smyrk TC et al (2015) Heterogenous MSH6 loss is a result of microsatellite instability within MSH6 and occurs in sporadic and hereditary colorectal and endometrial carcinomas. Am J Surg Pathol 39(10):1370–1376. https://doi.org/10.1097/PAS.0000000000000459
Tachon G, Frouin E, Karayan-Tapon L, Auriault ML, Godet J, Moulin V et al (2018) Heterogeneity of mismatch repair defect in colorectal cancer and its implications in clinical practice. Eur J Cancer 95:112–116. https://doi.org/10.1016/j.ejca.2018.01.087
McCarthy AJ, Capo-Chichi JM, Spence T, Grenier S, Stockley T, Kamel-Reid S et al (2019) Heterogenous loss of mismatch repair (MMR) protein expression: a challenge for immunohistochemical interpretation and microsatellite instability (MSI) evaluation. J Pathol Clin Res 5(2):115–129. https://doi.org/10.1002/cjp2.120
Joost P, Veurink N, Holck S, Klarskov L, Bojesen A, Harbo M et al (2014) Heterogenous mismatch-repair status in colorectal cancer. Diagn Pathol 9:126. https://doi.org/10.1186/1746-1596-9-126
Wu W, Liu Y, Zeng S, Han Y, Shen H (2021) Intratumor heterogeneity: the hidden barrier to immunotherapy against MSI tumors from the perspective of IFN-gamma signaling and tumor-infiltrating lymphocytes. J Hematol Oncol 14(1):160. https://doi.org/10.1186/s13045-021-01166-3
Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J et al (2019) Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 5(5):696–702. https://doi.org/10.1001/jamaoncol.2018.7098
Su D, Zhang D, Chen K, Lu J, Wu J, Cao X et al (2017) High performance of targeted next generation sequencing on variance detection in clinical tumor specimens in comparison with current conventional methods. J Exp Clin Cancer Res 36(1):121. https://doi.org/10.1186/s13046-017-0591-4
Yang N, Li Y, Liu Z, Qin H, Du D, Cao X et al (2018) The characteristics of ctDNA reveal the high complexity in matching the corresponding tumor tissues. BMC Cancer 18(1):319. https://doi.org/10.1186/s12885-018-4199-7
Peng H, Lu L, Zhou Z, Liu J, Zhang D, Nan K, et al. CNV detection from circulating tumor DNA in late stage non-small cell lung cancer patients. Genes (Basel). 2019;10(11). https://doi.org/10.3390/genes10110926
Wang Z, Zhao X, Gao C, Gong J, Wang X, Gao J, et al. Plasma-based microsatellite instability detection strategy to guide immune checkpoint blockade treatment. J Immunother Cancer. 2020;8(2). https://doi.org/10.1136/jitc-2020-001297
Wang Z, Wang X, Xu Y, Li J, Zhang X, Peng Z et al (2022) Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med 20(1):133. https://doi.org/10.1186/s12916-022-02327-y
Cristescu R, Lee J, Nebozhyn M, Kim KM, Ting JC, Wong SS et al (2015) Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 21(5):449–56. https://doi.org/10.1038/nm.3850
Olivier M, Hollstein M, Hainaut P (2010) TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol 2(1):a001008. https://doi.org/10.1101/cshperspect.a001008
Tahara T, Shibata T, Okamoto Y, Yamazaki J, Kawamura T, Horiguchi N et al (2016) Mutation spectrum of TP53 gene predicts clinicopathological features and survival of gastric cancer. Oncotarget. 7(27):42252–60. https://doi.org/10.18632/oncotarget.9770
Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, Liu SY et al (2017) Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 23(12):3012–24. https://doi.org/10.1158/1078-0432.CCR-16-2554
Li K, Luo H, Huang L, Luo H, Zhu X (2020) Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int. 20:16. https://doi.org/10.1186/s12935-019-1091-8
He WZ, Hu WM, Wang F, Rong YM, Yang L, Xie QK et al (2019) Comparison of mismatch repair status between primary and matched metastatic sites in patients with colorectal cancer. J Natl Compr Canc Netw. 17(10):1174–83. https://doi.org/10.6004/jnccn.2019.7308
Tong Y, Liu D, Zhang J (2020) Connection and distinction of tumor regression grading systems of gastrointestinal cancer. Pathol Res Pract. 216(9):153073. https://doi.org/10.1016/j.prp.2020.153073
Funding
This work was supported by Beijing Natural Science Foundation (Z200015 to XT.Z) and 2020 JingJian•TongShu Microsatellite Instability Research Foundation project (JJTS2020-004).
Author information
Authors and Affiliations
Contributions
All authors contributed to the research process. Manuscript preparation was performed by Xinyu Wang, Kang Jiang, Yajie Hu, Xinya Zhao, Lisha Yin, Xinting Diao, Xiuli Ma, Zan Yhang, Yu Xu, Yuezong Bai, Yu Sun, and Ziyu Li. Procedures and data analysis were performed by Xinyu Wang, and Kang Jiang. Drafting, conception, and design of the research was done by Yu Sun and Ziyu Li. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Human samples and clinical data were collected and used in accordance with the principles of the Declaration of Helsinki and approved by the Ethics committee of Peking University Cancer Hospital. All participants provided written informed consents.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Jiang, K., Hu, Y. et al. An exploration of gastric cancer with heterogeneous mismatch repair status. Virchows Arch 482, 517–523 (2023). https://doi.org/10.1007/s00428-023-03506-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03506-9