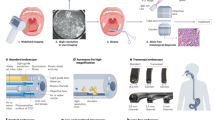
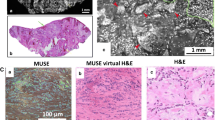
Abstract
We will briefly review the current paradigm and some recent developments in the area of clinical breast microscopy, highlighting several promising commercially available, and research-based platforms. Confocal microscopy (reflectance, fluorescence, and spectrally encoded), optical coherence tomography (wide field and full field), stereomicroscopy, open-top light sheet microscopy, microscopy with ultraviolet surface excitation, nonlinear microscopy, Raman scattering microscopy, photoacoustic microscopy, and needle microendoscopy will be discussed. Non-microscopic methods for breast pathology assessment are beyond the scope of this review. These microscopic technologies have to varying degrees the potential for transforming breast cancer care, but in order for any of these to be integrated into clinical practice there are several hurdles to overcome. In our review we will focus on what needs to be done in order for the commercially available technologies to become more established, what the technologies in the research domain need to do in order to reach the commercial realm; and finally, what the field of breast pathology might look like if these technologies were to be widely adopted.







Reproduced with permission from Hank Schmidt. Ref 24, Breast

Reproduced with permission from Shu Wang, Ref 25, Cancer

© The Optical Society, Biomed Opt Express

Reproduced with permission from Jonathan TC Liu. Ref 31, J Biomed Opt

Reproduced with permission from Dan Fu. Ref 36, Theranostics

Reproduced with permission from Lihong V Wang. Ref 38, Sci Adv
Similar content being viewed by others
References
No author listed 2021 US breast cancer statistics. Breastcancer.org. https://www.breastcancer.org/symptoms/understand_bc/statistics Accessed 22 April 2021
Metcalfe LN, Zysk AM, Yemul KS, Jacobs LK, Oker EE, Underwood HR (2017) Thompson AM 2017 Beyond the margins-economic costs and complications associated with repeated breast-conserving surgeries. JAMA Surg 152(11):1084–1086. https://doi.org/10.1001/jamasurg.2017.2661
Dillon MF, Hill AD, Quinn CM, O’Doherty A, McDermott EW (2005) O’Higgins N 2005 The accuracy of ultrasound, stereotactic, and clinical core biopsies in the diagnosis of breast cancer, with an analysis of false-negative cases. Ann Surg 242(5):701–707. https://doi.org/10.1097/01.sla.0000186186.05971.e0
Joe BN, Esserman LJ 2019 Breast biopsy. Uptodate. https://www-uptodate-com.treadwell.idm.oclc.org/contents/breast-biopsy?search=breast%20biopsy&source=search_result&selectedTitle=1~87&usage_type=default&display_rank=1 Accessed 22 April 2021
Maloney BW, McClatchy DM, Pogue BW, Paulsen KD, Wells WA, Barth RJ 2018 Review of methods for intraoperative margin detection for breast conserving surgery. J Biomed Opt 2018 Oct 2310 1 19 https://doi.org/10.1117/1.JBO.23.10.100901
Krishnamurthy S, Brown JQ, Iftimia N, Levenson RM, Rajadhyaksha M 2019 Ex vivo microscopy: a promising next-generation digital microscopy tool for surgical pathology practice Arch Pathol Lab Med 2019 143 9 1058 1068 https://doi.org/10.5858/arpa.2019-0058-RA.
Wells WA, Thrall M, Sorokina A, Fine J, Krishnamurthy S, Haroon A, Rao B, Shevchuk MM, Wolfsen HC, Tearney GJ, Hariri LP 2018 In Vivo and Ex Vivo Microscopy: Moving Toward the Integration of Optical Imaging Technologies Into Pathology Practice Arch Pathol Lab Med 2019 Mar 143 3288 298 https://doi.org/10.5858/arpa.2018-0298-RA
Fellers TJ, Davidson MW 2007Introduction to Confocal Microscopy. Olympus Fluoview Resource Center.http://www.olympusconfocal.com/theory/confocalintro.html. Accessed 22 April 2021
Ragazzi M, Longo C, Piana S 2016 Ex vivo (fluorescence) confocal microscopy in surgical pathology: state of the art. Adv Anat Pathol. 2016 23 3 159 169.https://doi.org/10.1097/PAP.0000000000000114
Clendenon SG, Young PA, Ferkowicz M, Phillips C (2011) Dunn KW 2011 Deep tissue fluorescent imaging in scattering specimens using confocal microscopy. Microsc Microanal 4(614):7. https://doi.org/10.1017/S1431927611000535
Yoshitake T, Giacomelli MG, Cahill LC, Schmolze DB, Vardeh H, Faulkner-Jones BE, Connolly JL (2016) Fujimoto JG 2016 Direct comparison between confocal and multiphoton microscopy for rapid histopathological evaluation of unfixed human breast tissue. J Biomed Opt 21(12):126021. https://doi.org/10.1117/1.JBO.21.12.126021
No author listed 2018)Vivascope 2500 ex-vivo confocal imaging data sheet. Caliber Imaging & Diagnostics, Inc. https://caliberid.com/2500-Datasheet.pdf. Accessed 22 April 2021
Dobbs JL, Ding H, Benveniste AP, Kuerer HM, Krishnamurthy S, Yang WT, Richards-Kortum RR 2013 28 October 2013. Feasibility of confocal fluorescence microscopy for real-time evaluation of neoplasia in fresh human breast tissue. J Biomed Opt 2013 Oct 1810 10601610.1117/1.JBO.18.10.106016
Dobbs JL, Mueller JL, Krishnamurthy S, Shin D, Kuerer H, Yang W, Ramanujam N, Richardson-Kortum R 2015 Micro-anatomical quantitative optical imaging: toward automated assessment of breast tissues. Breast Cancer Res. 2015 17 (1 105 https://doi.org/10.1186/s13058-015-0617-9
Dobbs J, Krishnamurthy S, Kyrish M, Benveniste AP, Yang W, Richards-Kortum R 92015) Confocal fluorescence microscopy for rapid evaluation of invasive tumor cellularity of inflammatory breast carcinoma core needle biopsies. Breast Cancer Res Treat. 2015 Jan 149 1 303 10. https://doi.org/10.1007/s10549-014-3182-5. Epub 2014 Nov 23 PMID: 25417171; PMCID: PMC4298669.
Abeytunge S, Larson BA, Peterson G, Morrow M, Rajadhyaksha M, Murray MP (2017) Evaluation of breast tissue with confocal strip-mosaicking microscopy: a test approach emulating pathology-like examination J Biomed Opt 22 3 034002 https://doi.org/10.1117/1.JBO.22.3.034002
No author listed 2019 Histolog scanner SamaTree Medical SA. https://samantree.com/product/. Accessed 22 April 2021
Elfgen C, Papassotiropoulos B, Varga Z, Moskovszky L, Nap M, Güth U, Baege A, Amann E, Chiesa F (2019) Tausch C (2019) Comparative analysis of confocal microscopy on fresh breast core needle biopsies and conventional histology. Diagn Pathol 14(1):58. https://doi.org/10.1186/s13000-019-0835-z
No autor listed 2020 Surgical margins Breastcancer org https://www.breastcancer.org/symptoms/diagnosis/margins Accessed 12 May 2021.
Skvara H, Plut U, Schmid JA, Jonak C (2012) Combining in vivo reflectance with fluorescence confocal microscopy provides additive information on skin morphology. Dermatology practical & conceptual 2(1):3–12. https://doi.org/10.5826/dpc.0201a02
Brachtel EF, Johnson NB, Huck AE, Rice-Stitt TL, Vangel MG, Smith BL, Tearney GJ (2016) Kang D (2016) Spectrally encoded confocal microscopy for diagnosing breast cancer in excision and margin specimens. Lab Invest 96(4):459–467. https://doi.org/10.1038/labinvest.2015.158
Fujimoto JG, Pitris C, Boppart SA, Brezinski ME 2000 Optical coherence tomography: an emerging technology for biomedical imaging and optical biopsy. Neoplasia. 2000 Jan Apr 2 1 2 9 25 https://doi.org/10.1038/sj.neo.7900071
Jodi Regts (2021) Perimeter medical imaging AI receives US FDA breakthrough device designation for its optical coherence tomography (OCT). Bloomberg. https://bloomberg.com/press-releases/2021-04-15/perimeter-medical-imaging-ai-receives-u-s-fda-breakthrough-device-designation-for-its-optical-coherence-tomography-oct Accessed 22 April 2021
Schmidt H, Connolly C, Jaffer S, Oza T, Weltz CR, Port ER (2020) Corben A (2020) Evaluation of surgically excised breast tissue microstructure using wide-field optical coherence tomography. Breast J 26(5):917–923. https://doi.org/10.1111/tbj.13663
Yang H, Zhang S, Liu P, Cheng L, Tong F, Liu H, Wang S, Liu M, Wang C, Peng Y, Xie F, Zhou B, Cao Y, Guo J, Zhang Y, Ma Y, Shen D, Xi P (2020) Wang S (2020) Use of high-resolution full-field optical coherence tomography and dynamic cell imaging for rapid intraoperative diagnosis during breast cancer surgery. Cancer 15(126 Suppl 16):3847–3856. https://doi.org/10.1002/cncr.32838
Nothngale PE, Chambers W, Davidson MW (no date listed) Introduction to stereomicroscopy. Nikon MicroscopyU. https://www.microscopyu.com/techniques/stereomicroscopy/introduction-to-stereomicroscopy Accessed 11 November 2021
Varga Z, Rageth C, Saurenmann E, Honegger C, von Orelli S, Fehr M, Fink D, Seifert B, Moch H, Caduff R (2008) Use of intraoperative stereomicroscopy for preventing loss of metastases during frozen sectioning of sentinel lymph nodes in breast cancer. Histopathology 52:597–604. https://doi.org/10.1111/j.1365-2559.2008.02998.x
Fadero TC, Gerbich TM, Rana K, Suzuki A, DiSalvo M, Schaefer KN, Heppert JK, Boothby TC, Goldstein B, Peifer M, Allbritton NL, Gladfelter AS, Maddox AS (2018) Maddox PS (2018) LITE microscopy: Tilted light-sheet excitation of model organisms offers high resolution and low photobleaching. J Cell Biol 217(5):1869–1882. https://doi.org/10.1083/jcb.201710087
Chen Y, Xie W, Glaser AK, Reder NP, Mao C, Dintzis SM, Vaughan JC (2019) Liu JTC (2019) Rapid pathology of lumpectomy margins with open-top light-sheet (OTLS) microscopy. Biomed Opt Express 10(3):1257–1272. https://doi.org/10.1364/BOE.10.001257
Glaser AK, Reder NP, Chen Y, McCarty EF, Yin C, Wei L, Wang Y, True LD (2017) Liu JTC (2017) Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens. Nat Biomed Eng 1(7):0084. https://doi.org/10.1038/s41551-017-0084
Xie W, Chen Y, Wang Y, Wei L, Yin C, Glaser AK, Fauver ME, Seibel EJ, Dintzis SM, Vaughan JC, Reder NP (2019) Liu JTC (2019) Microscopy with ultraviolet surface excitation for wide-area pathology of breast surgical margins. J Biomed Opt 24(2):1–11. https://doi.org/10.1117/1.JBO.24.2.026501
Fereidouni F, Harmany ZT, Tian M, Todd A, Kintner JA, McPherson JD, Borowsky AD, Bishop J, Lechpammer M, Demos SG (2017) Levenson R (2017) Microscopy with ultraviolet surface excitation for rapid slide-free histology. Nat Biomed Eng 1(12):957–966. https://doi.org/10.1038/s41551-017-0165-y
Balu M, Mikami H, Hou J, Potma EO, Tromberg BJ (2016) Large field of view multiphoton microscopy of human skin", Proc. SPIE 9712, Multiphoton Microscopy in the Biomedical Sciences XVI, 97121F (14 March 2016); https://doi.org/10.1117/12.2216163
Gubarkova EV, Elagin VV, Dudenkova VV, Kuznetsov SS, Karabut MM, Potapov AL, Vorontsov DA, Vorontsov AY, Sirotkina MA, Zagaynova EV (2021) Gladkova ND (2021) Multiphoton tomography in differentiation of morphological and molecular subtypes of breast cancer: A quantitative analysis. J Biophotonics 1:e202000471. https://doi.org/10.1002/jbio.202000471
Bhagavantam S(1971). Chandrasekhara Venkata Raman, 1888–1970. Biographical Memoirs of Fellows of the Royal Society. 1971 Nov 17: 564–592. https://doi.org/10.1098/rsbm.1971.0022.
Shin KS, Laohajaratsang M, Men S, Figueroa B, Dintzis SM (2020) Fu D (2020) Quantitative chemical imaging of breast calcifications in association with neoplastic processes. Theranostics 10(13):5865–5878. https://doi.org/10.7150/thno.43325
Zhang HF, Maslov K, Stoica G (2006) Wang LV (2006) Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat Biotechnol 24(7):848–851. https://doi.org/10.1038/nbt1220
Wong TTW, Zhang R, Hai P, Zhang C, Pleitez MA, Aft RL, Novack DV (2017 May 17) Wang LV (2017) Fast label-free multilayered histology-like imaging of human breast cancer by photoacoustic microscopy. Sci Adv 3(5):e1602168. https://doi.org/10.1126/sciadv.1602168
Chen CW, Blackwell TR, Naphas R, Winnard PTJ, Raman V, Glunde K, Chen Y (2009) Development of needle-based microendoscopy for fluorescence molecular imaging of breast tumor models. J Innov Opt Health Sci 2009 02(04):343–52. https://.doi.org/https://doi.org/10.1142/S1793545809000747
Krieger J et al 2020 Light sheet fluorescence microscopy. Wikipedia. https://en.wikipedia.org/wiki/Light_sheet_fluorescence_microscopy (article), https://commons.wikimedia.org/w/index.php?curid=26536357(figure). Accessed 22 April 2021
Taylor NP 2020FDA clears Spectra’s digital pathology module for primary diagnostics as US market heats up. MedTech Dive https://www.medtechdive.com/news/fda-clears-sectras-digital-pathology-module-for-primary-diagnostics-as-us/575346/Accessed May 11, 2021
Funding
Dr. Sgroi funded in part by the Breast Cancer Research Foundation (DCS).
Author information
Authors and Affiliations
Contributions
Dr. Lopez performed manuscript preparation, editing, proofreading, image preparation and permissions.
Dr. Sgroi done manuscript revision and editing.
Dr. Krishnamourthy contributed to manuscript revision, editing, and image preparation.
Dr. Tearney performed manuscript revision and editing.
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Krishnamurthy sponsored research agreements with Caliber ID and Perimeter Medical Imaging for investigator initiated research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lopez, D.R., Sgroi, D., Krishnamourthy, S. et al. Is Real-Time Microscopy on the Horizon? A Brief Review of the Potential Future Directions in Clinical Breast Tumor Microscopy Implementation. Virchows Arch 480, 211–227 (2022). https://doi.org/10.1007/s00428-022-03300-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03300-z