Abstract
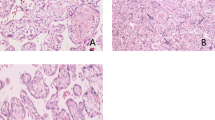
Transient abnormal myelopoiesis (TAM), also known as transient myeloproliferative disorder or transient leukemia, is a self-regressing neoplasia that afflicts infants with trisomy 21. A recent review article documented “myeloid cell thrombus (MCT)” and “fetal vascular malperfusion (FVM)” in placentas with TAM, although the characteristic TAM placental findings have not been clarified. Here, we compared the clinical and pathological placental findings between trisomy 21 patients with or without TAM. In 13 cases of trisomy 21, we identified six placentas with TAM and seven placentas without TAM. The six placentas with TAM included two stillborn cases. Microscopically, MCT was noted in all the cases, and a high incidence of FVM (50%) was observed in TAM cases. Immunohistochemically, MCT was found to be a platelet-rich thrombus. The placentas were grouped according to the presence or absence of TAM and subsequently compared. Clinically, the incidences of abnormal fetal heart rate pattern and fetal or neonatal death were significantly higher in TAM cases. Pathologically, placenta in TAM cases weighted more than those in cases without TAM, and the incidence of MCT was significantly higher in placentas with TAM. Moreover, the incidence of FVM was higher in placentas with TAM, but this difference was not statistically significant. We propose that MCT is a diagnostic feature of placentas with TAM and may be associated with poor fetal outcomes.


Similar content being viewed by others
References
Swerdlow SH, Campo E, Harris NL et al (2017) World Health Organization classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC Press, Lyon, pp 169–170
Foucar K, McKenna RW, Peterson LC, Kroft SH (2016) Tumors of the bone marrow, Armed Forces Institute of Pathology, 4th Series, Washington, DC, pp 144–148
Malinge S, Izraeli S, Crispino JD (2009) Insights into the manifestations, outcomes, and mechanisms of leukemogenesis in Down syndrome. Blood 113:2619–2628. https://doi.org/10.1182/blood-2008-11-163501
Qureshi F, Jacques SM, Johnson MP et al (1997) Trisomy 21 placentas: histopathological and immunohistochemical findings using proliferating cell nuclear antigen. Fetal Diagn Ther 12:210–215. https://doi.org/10.1159/000264470
Roberts L, Sebire NJ, Fowler D, Nicolaides KH (2000) Histomorphological features of chorionic villi at 10–14 weeks of gestation in trisomic and chromosomally normal pregnancies. Placenta 21:678–683. https://doi.org/10.1053/plac.2000.0553
Lentz SE, Coulson CC, Gocke CD, Fantaskey AP (1998) Placental pathology in maternal and neonatal myeloproliferative disorders. Obstet Gynecol 91:863. https://doi.org/10.1016/s0029-7844(98)00023-4
de Tar MW, Dittman W, Gilbert J (2000) Transient myeloproliferative disease of the newborn: case report with placental, cytogenetic, and flow cytometric findings. Hum Pathol 31:396–398. https://doi.org/10.1016/s0046-8177(00)80257-9
Heald B, Hilden JM, Zbuk K, Norton A et al (2007) Severe TMD/AMKL with GATA1 mutation in a stillborn fetus with Down syndrome. Nat Clin Pract Oncol 4:433–438. https://doi.org/10.1038/ncponc0876
Loh TJZ, Lian DWQ, Iyer P et al (2014) Congenital GATA1-mutated myeloproliferative disorder in trisomy 21 complicated by placental fetal thrombotic vasculopathy. Hum Pathol 45:2364–2367. https://doi.org/10.1016/j.humpath.2014.07.019
Dai Q, Reddy VVB, Choi JK, Faye-Petersen OM (2014) Trisomy 21-associated transient abnormal myelopoiesis involving the maternal space of the placenta: a case report and literature review. Pediatr Dev Pathol 17:366–373. https://doi.org/10.2350/14-04-1476-CR.1
Ravishankar S, Hoffman L, Lertsburapa T et al (2015) Extensive placental choriovascular infiltration by maturing myeloid cells in down syndrome-associated transient abnormal myelopoiesis. Pediatr Dev Pathol 18:231–236. https://doi.org/10.2350/14-11-1575-CR.1
Federmann B, Fasan A, Kagan KO, Haen S, Fend F (2015) Transient abnormal myelopoiesis/acute megakaryoblastic leukemia diagnosed in the placenta of a stillborn Down syndrome fetus with targeted next-generation sequencing. Leukemia 29:232–233. https://doi.org/10.1038/leu.2014.258
Yin L, Lovell MA, Wilson ML, Wei Q, Liang X (2016) Distinct GATA1 point mutations in monozygotic twins with Down syndrome and transient abnormal myelopoiesis from a triplet pregnancy: a case report and review of literature. Am J Clin Pathol 146:753–759. https://doi.org/10.1093/ajcp/aqw190
Kuo E, Kumarapeli AR (2020) Placental pathology in Down syndrome-associated transient abnormal myelopoiesis. Arch Pathol Lab Med 144:388–393. https://doi.org/10.5858/arpa.2018-0248-RS
Khong TY, Mooney EE, Ariel I et al (2016) Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group consensus statement. Arch Pathol Lab Med 140:698–713. https://doi.org/10.5858/arpa.2015-0225-CC
Macones GA, Hankins GD, Spong CY, Hauth J, Moore T (2008) The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol 112:661–666. https://doi.org/10.1097/AOG.0b013e3181841395
Sato Y, Maekawa K, Aman M et al (2019) CD39 downregulation in chronic intervillositis of unknown etiology. Virchows Arch 475:357–364. https://doi.org/10.1007/s00428-019-02598-6
Pinar H, Sung CJ, Oyer CE, Singer DB (1996) Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med 16:901–907. https://doi.org/10.1080/15513819609168713
Nakamura W, Goto H, Hayashi A et al (2020) Factors influencing platelet normalization of transient abnormal myelopoiesis. Pediatr Int 62:907–910. https://doi.org/10.1111/ped.14214
Yamato G, Park MJ, Sotomatsu M et al (2021) Clinical features of 35 Down syndrome patients with transient abnormal myelopoiesis at a single institution. Int J Hematol 113:662–667. https://doi.org/10.1007/s12185-020-03066-7
Fujihara I, Yanagisawa R, Fukushima Y et al (2016) Thrombocytosis in a newborn with Down syndrome and transient abnormal myelopoiesis. Br J Haematol 172:314. https://doi.org/10.1111/bjh.13808
Lee JW, Kim S, Jang PS, Chung NG, Cho B, Kim M (2021) Marked thrombocytosis resulting in pseudohyperkalemia in a neonate with transient abnormal myelopoiesis. Pediatr Blood Cancer 68:e28986. https://doi.org/10.1002/pbc.28986
Roberts DJ, Polizzano CP (2021) Atlas of placental pathology, Armed Forces Institute of Pathology, 5th Series, Washington, DC, pp 224–231
Redline RW, Ravishankar S (2018) Fetal vascular malperfusion, an update. APM.IS 126:561–569. doi: 10.1111/apm.12849.
Acknowledgements
We thank Takako Tokumitsu and Nahoko Udatsu for their excellent technical assistance. We also thank Editage (http://www.editage.jp/) for English language editing.
Funding
This study was supported by JP21Ko7775 from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
Tomimori K, Kodama Y, and Sato Y conceived and designed the study and wrote the manuscript. Tomimori K, Kodama Y, Doi K, and Katsuragi S collected the clinical data. Tanaka H, Yamashita A, Gi T, and Asada Y analyzed the histological data. Tomimori K and Sato Y wrote, edited, and reviewed the manuscript. All authors participated in the interpretation of the results and writing of the report and approved the final version submitted. Sato Y takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tomimori, K., Kodama, Y., Tanaka, H. et al. Myeloid cell thrombus and fetal vascular malperfusion in placentas with transient abnormal myelopoiesis. Virchows Arch 480, 1181–1187 (2022). https://doi.org/10.1007/s00428-022-03289-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-022-03289-5