Abstract
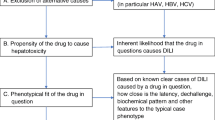
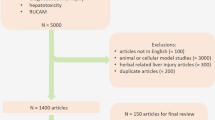
The clinical implications of the biopsy findings in cases of drug-induced liver injury (DILI) are not fully elucidated. The aim of this study was to evaluate the histopathological findings of cases diagnosed as DILI and to correlate them with clinical and biochemical findings (such as causality assessment algorithms). We searched our department database for all cases of liver biopsy with findings consistent with toxic liver disease and selected those with a clinical diagnosis of DILI. The causative relationships were established according to Roussel Uclaf Causality Assessment Method (RUCAM). A total of 53 cases of DILI were reviewed, most of them diagnosed in hospitalized patients (83%). The analytical toxicity profile was hepatocellular (R > 5) in 60% of the cases and cholestatic (R < 2) in 26.4% of cases. The group of drugs most implicated was the anti-microbials (18, 34%). The predominant histological patterns were “necroinflammation” (67.9%) and “cholestasis” (28.3%). The hepatocellular biochemical pattern was not associated with the presence of predominantly necroinflammatory findings in the biopsy (p = 0.44), and the biochemical cholestatic pattern was not associated with the presence of predominantly cholestatic findings in the biopsy (p = 0.51). This study supports that a better insight into the pathologic mechanisms associated with DILI should be based on liver biopsy due to the lack of a uniform correlation between clinical and biochemical patterns. Also, a liver biopsy may be used in those cases where clinical suspicion of DILI persists despite a low score on current causality assessment algorithms.


Similar content being viewed by others
References
Fontana RJ, Seeff LB, Andrade RJ, Bjornsson E, Day CP, Serrano J, Hoofnagle JH (2010) Standardization of nomenclature and causality assessment in drug-induced liver injury: summary of a clinical research workshop. Hepatology 52(2):730–742. https://doi.org/10.1002/hep.23696
Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, McCashland TM, Shakil AO, Hay JE, Hynan L, Crippin JS, Blei AT, Samuel G, Reisch J, Lee WM (2002) Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 137(12):947–954. https://doi.org/10.7326/0003-4819-137-12-200212170-00007
Temple RJ, Himmel MH (2002) Safety of newly approved drugs: implications for prescribing. Jama 287(17):2273–2275. https://doi.org/10.1001/jama.287.17.2273
Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH (2002) Timing of new black box warnings and withdrawals for prescription medications. Jama 287(17):2215–2220. https://doi.org/10.1001/jama.287.17.2215
Chalasani N, Bjornsson E (2010) Risk factors for idiosyncratic drug-induced liver injury. Gastroenterology 138(7):2246–2259. https://doi.org/10.1053/j.gastro.2010.04.001
Goodman ZD (2017) Phenotypes and pathology of drug-induced liver disease. Clin Liver Dis 21(1):89–101. https://doi.org/10.1016/j.cld.2016.08.006
Watkins PB, Seeff LB (2006) Drug-induced liver injury: summary of a single topic clinical research conference. Hepatology 43(3):618–631. https://doi.org/10.1002/hep.21095
Teschke R, Schwarzenboeck A, Hennermann KH (2008) Kava hepatotoxicity: a clinical survey and critical analysis of 26 suspected cases. Eur J Gastroenterol Hepatol 20(12):1182–1193. https://doi.org/10.1097/MEG.0b013e3283036768
Kleiner DE, Chalasani NP, Lee WM, Fontana RJ, Bonkovsky HL, Watkins PB, Hayashi PH, Davern TJ, Navarro V, Reddy R, Talwalkar JA, Stolz A, Gu J, Barnhart H, Hoofnagle JH (2014) Hepatic histological findings in suspected drug-induced liver injury: systematic evaluation and clinical associations. Hepatology 59(2):661–670. https://doi.org/10.1002/hep.26709
Kleiner DE (2014) Liver histology in the diagnosis and prognosis of drug-induced liver injury. Clin Liver Dis 4(1):12–16. https://doi.org/10.1002/cld.371
Katoonizadeh A, Nevens F, Verslype C, Pirenne J, Roskams T (2006) Liver regeneration in acute severe liver impairment: a clinicopathological correlation study. Liver Int 26(10):1225–1233. https://doi.org/10.1111/j.1478-3231.2006.01377.x
Bjornsson E, Kalaitzakis E, Olsson R (2007) The impact of eosinophilia and hepatic necrosis on prognosis in patients with drug-induced liver injury. Aliment Pharmacol Ther 25(12):1411–1421. https://doi.org/10.1111/j.1365-2036.2007.03330.x
Kleiner DE (2011) Liver injury due to drugs and herbal agents. In: Saxena R (ed) Practical hepatic pathology. 1st edition edn. Sanders, Philadelphia, pp 311–352
Batts KP, Ludwig J (1995) Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 19(12):1409–1417
EASL Clinical Practice Guidelines: Autoimmune hepatitis (2015). J Hepatol 63 (4):971–1004. doi:https://doi.org/10.1016/j.jhep.2015.06.030
EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis (2017). J Hepatol 67 (1):145–172. doi:https://doi.org/10.1016/j.jhep.2017.03.022
Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, Hunt CM, Wilke RA, Avigan M, Kaplowitz N, Bjornsson E, Daly AK (2011) Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther 89(6):806–815. https://doi.org/10.1038/clpt.2011.58
Benichou C (1990) Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol 11(2):272–276
Bjornsson E (2006) Drug-induced liver injury: Hy’s rule revisited. Clin Pharmacol Ther 79(6):521–528. https://doi.org/10.1016/j.clpt.2006.02.012
Reuben A (2004) Hy’s law. Hepatology 39(2):574–578. https://doi.org/10.1002/hep.20081
Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WGE, Czaja AJ, Desmet VJ, Donaldson PT, Eddleston ALWF, Fainboim L, Heathcote J, Homberg JC, Hoofnagle JH, Kakumu S, Krawitt EL, Mackay IR, MacSween RNM, Maddrey WC, Manns MP, McFarlane IG, Meyer zum Büschenfelde KH, Mieli-Vergani G, Nakanuma Y, Nishioka M, Penner E, Porta G, Portmann BC, Reed WD, Rodes J, Schalm SW, Scheuer PJ, Schrumpf E, Seki T, Toda G, Tsuji T, Tygstrup N, Vergani D, Zeniya M (1999) International Autoimmune Hepatitis Group report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 31(5):929–938. https://doi.org/10.1016/S0168-8278(99)80297-9
EASL Clinical Practice Guidelines: Drug-induced liver injury (2019). J Hepatol 70 (6):1222–1261. doi:https://doi.org/10.1016/j.jhep.2019.02.014
Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD (2009) Liver biopsy. Hepatology 49(3):1017–1044. https://doi.org/10.1002/hep.22742
Gasmi B, Kleiner DE (2020) Liver histology: diagnostic and prognostic features. Clin Liver Dis 24(1):61–74. https://doi.org/10.1016/j.cld.2019.09.004
Becker MW, Lunardelli MJM, Tovo CV, Blatt CR (2019) Drug and herb-induced liver injury: a critical review of Brazilian cases with proposals for the improvement of causality assessment using RUCAM. Ann Hepatol 18(5):742–750. https://doi.org/10.1016/j.aohep.2019.03.010
Teschke R, Frenzel C (2013) Drug induced liver injury: do we still need a routine liver biopsy for diagnosis today? Ann Hepatol 13(1):121–126
Hayashi PH (2009) Causality assessment in drug-induced liver injury. Semin Liver Dis 29(4):348–356. https://doi.org/10.1055/s-0029-1240003
Author information
Authors and Affiliations
Contributions
Pedro Costa-Moreira was responsible for the study design, acquisition, and interpretation of data, drafting the manuscript, and statistical analysis. Rui Gaspar, Pedro Pereira, Susana Lopes, Pedro Canão, and Joanne Lopes were responsible for the acquisition and interpretation of data. Fátima Carneiro and Guilherme Macedo were responsible for critical revision of the manuscript for relevant intellectual content.
Corresponding author
Ethics declarations
This study was conducted according to the Declaration of Helsinki. The ethical approval for this study was obtained from the Ethics Committee of “Centro Hospitalar e Universitário São João”/Faculty of Medicine of the Porto University. Informed consent for research use of the histopathological data was obtained from each patient before the liver biopsy was performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Costa-Moreira, P., Gaspar, R., Pereira, P. et al. Role of liver biopsy in the era of clinical prediction scores for “drug-induced liver injury” (DILI): experience of a tertiary referral hospital. Virchows Arch 477, 517–525 (2020). https://doi.org/10.1007/s00428-020-02824-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02824-6