Abstract
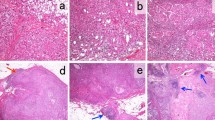
The immune system plays a key role in tumor surveillance and escape. Recently, CD73 has been proposed as a prognostic biomarker associated with disease-free survival and overall survival in triple negative breast cancer (TNBC). In this study, we investigated the role of both CD73 expression and stromal tumor–infiltrating lymphocytes (TILs) in predicting the pathologic response of TNBC to neoadjuvant chemotherapy (NACT). We retrospectively analyzed CD73 immunohistochemical expression and stromal TILs on 61 consecutive biopsies from patients who received standard NACT. Twenty-three patients (38%) achieved pathologic complete response (pCR). TILs were present in the majority of biopsies (93%) with percentages ranging from 2 to 80%. High TILs (≥ 50%) were found in 30% of cases, and in this group, pCR was achieved in 76.5% of cases. Levels of TILs were associated with a better pathologic response only at univariate analysis (p = 0.037). The median value of CD73 expression on tumor cells was 40%. In 32 (52.5%) basal biopsies, CD73 expression was below or equal to median value (“low CD73”). A pCR was obtained in 53% of cases with “low CD73” and in 21% with high CD73, and this was statistically different both at univariate (p = 0.011) and multivariate (p = 0.014) analysis.
Our results suggest that CD73 expression better predicts the response to NACT than TILs in TNBC. Characterization of both TILs and microenvironment could be a promising approach to personalize treatment.


Similar content being viewed by others
References
Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong YN, Blayney DW, Niland JC, Winer EP, Weeks JC (2012) Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 118(22):5463–5472. https://doi.org/10.1002/cncr.27581
Santonja A, Sánchez-Muñoz A, Lluch A et al (2018) Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget 9(41):26406–26416. https://doi.org/10.18632/oncotarget.25413
Matsumoto H, Koo SL, Dent R, Tan PH, Iqbal J (2015) Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol 68:506–510. https://doi.org/10.1136/jclinpath-2015-202944139
Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G (2011) Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 71:5601–5605. https://doi.org/10.1158/0008-5472.CAN-11-1316
Ono M, Tsuda H, Shimizu C et al (2012) Tumor-infiltrating lymphocytes are correlated with response to neoadjuvant chemotherapy in triple-negative breast cancer. Breast Cancer Res Treat 132:793–805. https://doi.org/10.1007/s10549-011-1554-7
Beckers RK, Selinger CI, Vilain R et al (2016) Programmed death ligand 1 expression in triple-negative breast cancer is associated with tumour-infiltrating lymphocytes and improved outcome. Histopathology 69:25–34. https://doi.org/10.1111/his.12904
Sabatier R, Finetti P, Mamessier E et al (2015) Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget 7:5449–5469
Schmid P, Adams S, Rugo HS et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. https://doi.org/10.1056/NEJMoa1809615
Adams S, Loi S, Toppmeyer D et al (2017) Phase 2 study of pembrolizumab as first-line therapy for PD-L1–positive metastatic triple-negative breast cancer (mTNBC): preliminary data from KEYNOTE-086 cohort B. J Clin Oncol 35 [suppl; abstr 1088].
Nanda R, Chow LQ, Dees EC et al (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012Study. J Clin Oncol 34:2460–2467. https://doi.org/10.1200/JCO.2015.64.8931
Inoue Y, Yoshimura K, Kurabe N et al (2017) Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget 8(5):8738–8751. https://doi.org/10.18632/oncotarget.14434
Leclerc BG, Charlebois R, Chouinard G, Allard B, Pommey S, Saad F, Stagg J (2016) CD73 expression is an independent prognostic factor in prostate cancer. Clin Cancer Res 22:158–166. https://doi.org/10.1158/1078-0432.CCR-15-1181
Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J (2013) CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 110:11091–11096. https://doi.org/10.1073/pnas.1222251110
Wu X, He X, Chen Y et al (2012) High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol 106:130–137. https://doi.org/10.1002/jso.23056
Jiang T, Xu X, Qiao M et al (2018) Comprehensive evaluation of NT5E/CD73 expression and its prognostic significance in distinct types of cancers. BMC Cancer 18(1):267. https://doi.org/10.1186/s12885-018-4073-7
Allard D, Turcotte M, Stagg J (2017) Targeting A2 adenosine receptors in cancer. Immunol Cell Biol 95(4):333–339. https://doi.org/10.1038/icb.2017.8
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16:177–192. https://doi.org/10.1038/nri.2016.4
Sitkovsky MV, Hatfield S, Abbott R et al (2014) Hostile, hypoxia-A2- adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res 2(7):598–605. https://doi.org/10.1158/2326-6066.CIR-14-0075
Mittal D, Sinha D, Barkauskas D et al (2016) Adenosine 2B receptor expression on cancer cells promotes metastasis. Cancer Res 76(15):4372–4382. https://doi.org/10.1158/0008-5472.CAN-16-0544
Allard D, Allard B, Gaudreau PO et al (2016) CD73-adenosine: a next-generation target in immuno-oncology. Immunotherapy 8:145–163. https://doi.org/10.2217/imt.15.106
Allard B, Longhi MS, Robson SC, Stagg J (2017) The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 276:121–144. https://doi.org/10.1111/imr.12528
Deaglio S, Dwyer KM, Gao W et al (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 204:1257–1265
Leone RD, Emens LA (2018) Targeting adenosine for cancer immunotherapy. J Immunother Cancer 6(1):57. https://doi.org/10.1186/s40425-018-0360-8
Supernat A, Markiewicz A, Welnicka-Jaskiewicz M et al (2012) CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol 20:103–107
Salgado R, Denkert C, Demaria S et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271. https://doi.org/10.1093/annonc/mdu450
Pinder SE, Rakha EA, Purdie CA et al (2015) Translational Subgroup of the NCRI Breast Clinical Studies Group. Macroscopic handling and reporting of breast cancer specimens pre- and post-neoadjuvant chemotherapy treatment: review of pathological issues and suggested approaches. Histopathology 67(3):279–293. https://doi.org/10.1111/his.12649
Provenzano E, Bossuyt V, Viale G et al (2015) Residual Disease Characterization Working Group of the Breast International Group-North American Breast Cancer Group Collaboration. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol 28(9):1185–1201. https://doi.org/10.1038/modpathol.2015.74
Edge SB, Compton CC (2010) The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17(6):1471–1474. https://doi.org/10.1245/s10434-010-0985-4
Elston CW, Ellis IO (1991 Nov) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 19(5):403–410
Cortazar P, Zhang LJ, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Yamaguchi R, Tanaka M, Yano A et al (2012) Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Human Pathology 43(10):1688–1694. https://doi.org/10.1016/j.humpath.2011.12.013
Cerbelli B, Pernazza A, Botticelli A et al (2017) PD-L1 expression in TNBC: a predictive biomarker of response to neoadjuvant chemotherapy? Biomed Res Int 2017:1750925. https://doi.org/10.1155/2017/1750925
Denkert C, von Minckwitz G, Darb-Esfahani S et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19(1):40–50. https://doi.org/10.1016/S1470-2045(17)30904-X
Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Haskó G (2016) Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends Cancer 2(2):95–109
Buisseret L, Pommey S, Allard B et al (2018) Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann Oncol 29(4):1056–1062. https://doi.org/10.1093/annonc/mdx730
Samanta D, Park Y, Ni X et al (2018) Chemotherapy induces enrichment of CD47+/CD73+/PDL1+ immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A 115(6):E1239–E1248. https://doi.org/10.1073/pnas.1718197115
Adams S, Loi S, Toppmeyer D et al (2019) Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30(3):405–411. https://doi.org/10.1093/annonc/mdy518
Schmid P, Park YE, Muñoz-Couselo E et al Pembrolizumab (pembro) + chemotherapy (chemo) as neoadjuvant treatment for triple negative breast cancer (TNBC): Preliminary results from KEYNOTE-173. JCO.2017.35.15_suppl.556.
Loibl S, Untch M, Burchardi N et al (2018) Randomized phase II neoadjuvant study (GeparNuevo) to investigate the addition of durvalumab to a taxane-anthracycline containing chemotherapy in triple negative breast cancer (TNBC). J Clin Oncol 36(15_suppl):104–104
Siu LL, Howard Burris H, Le DT et al Abstract CT180: preliminary phase 1 profile of BMS-986179, an anti-CD73 antibody, in combination with nivolumab in patients with advanced solid tumors. doi: https://doi.org/10.1158/1538-7445.AM2018-CT180
Contributions
Cerbelli Bruna contributed to design of work, data analysis, and manuscript writing;
Botticelli Andrea contributed to design of work, data analysis, and manuscript writing;
Pisano Annalinda contributed to acquisition and analysis of data;
Pernazza Angela contributed to interpretation of data;
Campagna Domenico contributed to acquisition of data;
De Luca Alessandro contributed to acquisition of data;
Ascierto Paolo Antonio revised the work critically for important intellectual content;
Pignataro Maria Gemma contributed to acquisition and analysis of data;
Pelullo Maria contributed to analysis of data;
Della Rocca Carlo revised the work critically for important intellectual content;
Marchetti Paolo revised and approved the final version manuscript;
Fortunato Lucio contributed to the conception of the work and critically revised it;
Costarelli Leopoldo contributed to the conception of the work and critically revised it;
d’Amati Giulia contributed to the design of the work, data analysis, and manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study complies with ethical standards with both Institutions. All patients gave their informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Rights and permissions
About this article
Cite this article
Cerbelli, B., Botticelli, A., Pisano, A. et al. CD73 expression and pathologic response to neoadjuvant chemotherapy in triple negative breast cancer. Virchows Arch 476, 569–576 (2020). https://doi.org/10.1007/s00428-019-02722-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02722-6