Abstract
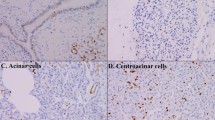
Aberrant Fhit expression characterizes a large proportion of primary pancreatic ductal adenocarcinomas (PDACs), but fragmentary information is available on Fhit expression during the phenotypic changes of pancreatic ductal epithelium during multistep transformation. We assessed Fhit expression by immunohistochemistry in two different multistep pancreatic carcinogenic processes: pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasia (IPMN). We considered 105 surgically treated PDACs/IPMNs and selected 30 samples of non-neoplastic pancreatic parenchyma, 50 PanIN lesions, 30 IPMNs, 15 IPMNs with associated invasive carcinoma, and 60 adenocarcinomas. Normal pancreatic ducts and surrounding acinar cells consistently showed moderate to strong Fhit immunoreactivity. Significant down-regulation of Fhit expression was observed in association with increasing severity of dysplastia/neoplastia in both carcinogenic processes. This was further confirmed by studying multiple lesions obtained from the same surgical specimen. Of 60 PDACs, only 14 showed Fhit expression comparable to normal pancreatic ductal epithelium, while the remainder (77%) showed clearly negative or reduced Fhit expression. This study demonstrates that Fhit down-regulation is an early event in both multistep carcinogenic processes leading to PDAC.



Similar content being viewed by others
References
Hidalgo M (2010) Pancreatic cancer. N Engl J Med 362:1605–1617
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC, Nourse C, Murtaugh LC, Harliwong I, Idrisoglu S, Manning S, Nourbakhsh E, Wani S, Fink L, Holmes O, Chin V, Anderson MJ, Kazakoff S, Leonard C, Newell F, Waddell N, Wood S, Xu Q, Wilson PJ, Cloonan N, Kassahn KS, Taylor D, Quek K, Robertson A, Pantano L, Mincarelli L, Sanchez LN, Evers L, Wu J, Pinese M, Cowley MJ, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chantrill LA, Mawson A, Humphris J, Chou A, Pajic M, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Lovell JA, Merrett ND, Toon CW, Epari K, Nguyen NQ, Barbour A, Zeps N, Moran-Jones K, Jamieson NB, Graham JS, Duthie F, Oien K, Hair J, Grutzmann R, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Rusev B, Capelli P, Salvia R, Tortora G, Mukhopadhyay D, Petersen GM, Australian Pancreatic Cancer Genome Initiative, Munzy DM, Fisher WE, Karim SA, Eshleman JR, Hruban RH, Pilarsky C, Morton JP, Sansom OJ, Scarpa A, Musgrove EA, Bailey UM, Hofmann O, Sutherland RL, Wheeler DA, Gill AJ, Gibbs RA, Pearson JV, Waddell N, Biankin AV, Grimmond SM (2016) Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531:47–52
Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Australian Pancreatic Cancer Genome Initiative, Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LF, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM (2012) Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 491:399–405
Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (2008) Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321:1801–1806
Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) World health organization classification of tumours of the digestive system
Gnoni A, Licchetta A, Scarpa A, Azzariti A, Brunetti AE, Simone G, Nardulli P, Santini D, Aieta M, Delcuratolo S, Silvestris N (2013) Carcinogenesis of pancreatic adenocarcinoma: precursor lesions. Int J MolSci 14:19731–19762
Koorstra JB, Hustinx SR, Offerhaus GJ, Maitra A (2008) Pancreatic carcinogenesis. Pancreatology 8:110–125
Adsay V, Mino-Kenudson M, Furukawa T, Basturk O, Zamboni G, Marchegiani G, Bassi C, Salvia R, Malleo G, Paiella S, Wolfgang CL, Matthaei H, Offerhaus GJ, Adham M, Bruno MJ, Reid MD, Krasinskas A, Kloppel G, Ohike N, Tajiri T, Jang KT, Roa JC, Allen P, Fernandez-del Castillo C, Jang JY, Klimstra DS, Hruban RH, Members of Verona Consensus Meeting, 2013 (2016) Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: recommendations of Verona consensus meeting. Ann Surg 263:162–177
Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Kloppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T, Baltimore Consensus Meeting (2015) A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 39:1730–1741
Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, Hruban RH, Maitra A, Kinzler K, Vogelstein B, Goggins M (2012) Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 142:730–733.e9
Murphy SJ, Hart SN, Lima JF, Kipp BR, Klebig M, Winters JL, Szabo C, Zhang L, Eckloff BW, Petersen GM, Scherer SE, Gibbs RA, McWilliams RR, Vasmatzis G, Couch FJ (2013) Genetic alterations associated with progression from pancreatic intraepithelial neoplasia to invasive pancreatic tumor. Gastroenterology 145:1098–1109.e1
Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-Del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA Jr, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM (2015) A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology 149:1501–1510
Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, Goggins M, Canto MI, Schulick RD, Edil BH, Wolfgang CL, Klein AP, Diaz LA Jr, Allen PJ, Schmidt CM, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B (2011a) Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. SciTransl Med 3:92ra66
Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B (2011b) Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 108:21188–21193
Huebner K (2011) Molecular biology: DNA fragility put into context. Nature 470:46–47
Huebner K, Garrison PN, Barnes LD, Croce CM (1998) The role of the FHIT/FRA3B locus in cancer. Annu Rev Genet 32:7–31
Ohta M, Inoue H, Cotticelli MG, Kastury K, Baffa R, Palazzo J, Siprashvili Z, Mori M, McCue P, Druck T, Croce CM, Huebner K (1996) The FHIT gene, spanning the chromosome 3p14.2 fragile site and renal carcinoma-associated t(3;8) breakpoint, is abnormal in digestive tract cancers. Cell 84:587–597
Birnbaum DJ, Adelaide J, Mamessier E, Finetti P, Lagarde A, Monges G, Viret F, Goncalves A, Turrini O, Delpero JR, Iovanna J, Giovannini M, Birnbaum D, Chaffanet M (2011) Genome profiling of pancreatic adenocarcinoma. Genes Chromosomes Cancer 50:456–465
Sorio C, Baron A, Orlandini S, Zamboni G, Pederzoli P, Huebner K, Scarpa A (1999) The FHIT gene is expressed in pancreatic ductular cells and is altered in pancreatic cancers. Cancer Res 59:1308–1314
Shridhar R, Shridhar V, Wang X, Paradee W, Dugan M, Sarkar F, Wilke C, Glover TW, Vaitkevicius VK, Smith DI (1996) Frequent breakpoints in the 3p14.2 fragile site, FRA3B, in pancreatic tumors. Cancer Res 56:4347–4350
Hilgers W, Groot Koerkamp B, Geradts J, Tang DJ, Yeo CJ, Hruban RH, Kern SE (2000) Genomic FHIT analysis in RER+ and RER- adenocarcinomas of the pancreas. Genes Chromosomes Cancer 27:239–243
Murphy SJ, Hart SN, Halling GC, Johnson SH, Smadbeck JB, Drucker T, Lima JF, Rohakhtar FR, Harris FR, Kosari F, Subramanian S, Petersen GM, Wiltshire TD, Kipp BR, Truty MJ, McWilliams RR, Couch FJ, Vasmatzis G (2016) Integrated genomic analysis of pancreatic ductal adenocarcinomas reveals genomic rearrangement events as significant drivers of disease. Cancer Res 76:749–761
Bloomston M, Kneile J, Butterfield M, Dillhoff M, Muscarella P, Ellison EC, Melvin WS, Croce CM, Pichiorri F, Huebner K, Frankel WL (2009) Coordinate loss of fragile gene expression in pancreatobiliary cancers: correlations among markers and clinical features. Ann Surg Oncol 16:2331–2338
Simon B, Bartsch D, Barth P, Prasnikar N, Munch K, Blum A, Arnold R, Goke B (1998) Frequent abnormalities of the putative tumor suppressor gene FHIT at 3p14.2 in pancreatic carcinoma cell lines. Cancer Res 58:1583–1587
Dumon KR, Ishii H, Vecchione A, Trapasso F, Baldassarre G, Chakrani F, Druck T, Rosato EF, Williams NN, Baffa R, During MJ, Huebner K, Croce CM (2001) Fragile histidine triad expression delays tumor development and induces apoptosis in human pancreatic cancer. Cancer Res 61:4827–4836
Cao J, Chen XP, Li WL, Xia J, Du H, Tang WB, Wang H, Chen XW, Xiao HQ, Li YY (2007) Decreased fragile histidine triad expression in colorectal cancer and its association with apoptosis inhibition. World J Gastroenterol 13:1018–1026
Sozzi G, Pastorino U, Moiraghi L, Tagliabue E, Pezzella F, Ghirelli C, Tornielli S, Sard L, Huebner K, Pierotti MA, Croce CM, Pilotti S (1998) Loss of FHIT function in lung cancer and preinvasive bronchial lesions. Cancer Res 58:5032–5037
Terry G, Ho L, Londesborough P, Duggan C, Hanby A, Cuzick J (2007) The expression of FHIT, PCNA and EGFR in benign and malignant breast lesions. Br J Cancer 96:110–117
Saldivar JC, Miuma S, Bene J, Hosseini SA, Shibata H, Sun J, Wheeler LJ, Mathews CK, Huebner K (2012) Initiation of genome instability and preneoplastic processes through loss of Fhit expression. PLoS Genet 8:e1003077
Pichiorri F, Palumbo T, Suh SS, Okamura H, Trapasso F, Ishii H, Huebner K, Croce CM (2008) Fhit tumor suppressor: guardian of the preneoplastic genome. Future Oncol 4:815–824
Sorio C, Capelli P, Lissandrini D, Moore PS, Balzarini P, Falconi M, Zamboni G, Scarpa A (2005) Mucinous cystic carcinoma of the pancreas: a unique cell line and xenograft model of a preinvasive lesion. Virchows Arch 446:239–245
Brozzetti S, French D, Polistena A, Di Marzo L, Pisani T, Marchese R, Mingazzini P, Mascioli G, Vecchione A, Cavallaro A (2002) Papillary solid and cystic pancreatic tumor. Genetic prediction factors for malignancy: report of three cases. Anticancer Res 22:2341–2346
Amato E, Molin MD, Mafficini A, Yu J, Malleo G, Rusev B, Fassan M, Antonello D, Sadakari Y, Castelli P, Zamboni G, Maitra A, Salvia R, Hruban RH, Bassi C, Capelli P, Lawlor RT, Goggins M, Scarpa A (2014) Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 233:217–227
Guler G, Uner A, Guler N, Han SY, Iliopoulos D, Hauck WW, McCue P, Huebner K (2004) The fragile genes FHIT and WWOX are inactivated coordinately in invasive breast carcinoma. Cancer 100:1605–1614
Luchini C, Parcesepe P, Mafficini A, Nottegar A, Parolini C, Veronese N, Remo A, Manfrin E (2015) Specific expression patterns of epithelial to mesenchymal transition factors in gestational molar disease. Placenta 36:1318–1324
Luchini C, Parcesepe P, Nottegar A, Parolini C, Mafficini A, Remo A, Chilosi M, Manfrin E (2016) CD71 in gestational pathology: a versatile immunohistochemical marker with new possible applications. Appl Immunohistochem MolMorphol 24:215–220
Bragantini E, Barbi S, Beghelli S, Moore PS, de Manzoni G, Roviello F, Tomezzoli A, Vindigni C, Baffa R, Scarpa A (2006) Loss of Fhit expression is associated with poorer survival in gastric cancer but is not an independent prognostic marker. J Cancer Res Clin Oncol 132:45–50
Detre S, Saclani Jotti G, Dowsett M (1995) A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol 48:876–878
Tsujiuchi T, Sasaki Y, Kubozoe T, Konishi Y, Tsutsumi M (2003) Alterations in the Fhit gene in pancreatic duct adenocarcinomas induced by N-nitrosobis(2-oxopropyl)amine in hamsters. MolCarcinog 36:60–66
Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Orntoft T, Lukas J, Bartek J (2005) DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434:864–870
Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD (2005) Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434:907–913
Karras JR, Schrock MS, Batar B, Zhang J, La Perle K, Druck T, Huebner K (2016) Fhit loss-associated initiation and progression of neoplasia in vitro. Cancer Sci 107:1590–1598
Tomizawa Y, Nakajima T, Kohno T, Saito R, Yamaguchi N, Yokota J (1998) Clinicopathological significance of Fhit protein expression in stage I non-small cell lung carcinoma. Cancer Res 58:5478–5483
Fassan M, Baffa R, Kiss A (2013) Advanced precancerous lesions within the GI tract: the molecular background. Best Pract Res Clin Gastroenterol 27:159–169
Mafficini A, Amato E, Fassan M, Simbolo M, Antonello D, Vicentini C, Scardoni M, Bersani S, Gottardi M, Rusev B, Malpeli G, Corbo V, Barbi S, Sikora KO, Lawlor RT, Tortora G, Scarpa A (2014) Reporting tumor molecular heterogeneity in histopathological diagnosis. PLoS One 9:e104979
Amosenko FA, Kazubskaia TP, Gromyko OE, Matveeva TI, Korchagina EL, Nasedkina TV, Gar'kavtseva RF, Kalinin VN (2009) Analysis of K-ras, BRCA1/2, CHEK2 mutations and microsatellite markers (loss of heterozygosity at 9p, 17p and 18q) in sporadic pancreas adenocarcinomas. MolBiol (Mosk) 43:414–421
Baumgart M, Werther M, Bockholt A, Scheurer M, Ruschoff J, Dietmaier W, Ghadimi BM, Heinmoller E (2010) Genomic instability at both the base pair level and the chromosomal level is detectable in earliest PanIN lesions in tissues of chronic pancreatitis. Pancreas 39:1093–1103
Matsuda Y, Ishiwata T, Izumiyama-Shimomura N, Hamayasu H, Fujiwara M, Tomita K, Hiraishi N, Nakamura K, Ishikawa N, Aida J, Takubo K, Arai T (2015) Gradual telomere shortening and increasing chromosomal instability among PanIN grades and normal ductal epithelia with and without cancer in the pancreas. PLoS One 10:e0117575
van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, Offerhaus GJ, Hicks JL, Wilentz RE, Goggins MG, De Marzo AM, Hruban RH, Maitra A (2002) Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol 161:1541–1547
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The materials used have been collected under Program 853 protocol 298CE on 15 February 2002 and Program 1885 protocol 52438 on 23 November 2010. The protocols include informed consent of the patient and were approved by the local ethics committee of the Integrated University Hospital Trust of Verona. The first approval (prog. 853) regarded the collection of pancreas samples for use in molecular research studies. This was later updated (prog. 1885) for the creation of a coordinated biobank for the collection of samples from all cancer patients that included neoplastic and associated local and distant normal tissue. The approved programs include tissue processing and storage methods of FFPE tissues of both neoplastic and normal tissues.
Funding
This work was supported by AssociazioneItalianaRicercaCancro (AIRC grant n. 12182, to AS); Cam-Pac FP7 Grant no: 602783 (to AS); Italian Cancer Genome Project (FIRB RBAP10AHJB to AS); FIMP-Italian Ministry of Health (CUP_J33G13000210001 to AS), and USPHS National Cancer Institute grant CA120516 (to KH).
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Aldo Scarpa and Kay Huebner are co-last authors.
Rights and permissions
About this article
Cite this article
Fassan, M., Rusev, B., Corbo, V. et al. Fhit down-regulation is an early event in pancreatic carcinogenesis. Virchows Arch 470, 647–653 (2017). https://doi.org/10.1007/s00428-017-2105-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2105-3