Abstract
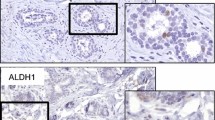
Secreted protein acidic and rich in cysteine (SPARC) plays an essential role in tumor invasion and metastasis. The present work was undertaken to detect expression of SPARC mRNA in phyllodes tumors (PTs) and its association with SPARC protein expression. This study also evaluated expression of SPARC mRNA and its correlation between grade and clinical behavior of PTs. In addition, we assessed in PTs the association of expression of SPARC with that of matrix metalloproteinase (MMP)-2 and of MMP-9. SPARC mRNA expression was determined by RNAscope in situ hybridization (ISH) in 50 benign, 22 borderline, and 10 malignant PTs using a tissue microarray. Furthermore, we applied immunohistochemistry (IHC) to examine expression of SPARC, MMP-2, and MMP-9. SPARC mRNA appeared to be concentrated mainly in the stromal compartment of PTs. IHC staining patterns of SPARC protein showed concordance with SPARC mRNA ISH results. Stromal SPARC expression increased continuously as PTs progress from benign through borderline to malignant PTs, both at mRNA (using ISH) (P = 0.044) and protein level (using IHC) (P = 0.000). The recurrence percentage was higher in the stromal SPARC mRNA or protein-positive group than in the SPARC-negative group but this difference was not statistically significant. Stromal SPARC mRNA and protein expression was associated with PT grade and correlated with MMP-2 expression. These results indicate that SPARC-mediated degradation of the extracellular matrix, and its possible association with MMPs, might contribute to progression of PTs.



Similar content being viewed by others
References
Rosen PP (2009) Fibroepithelial neoplasms. In: Rosen PP (ed) Rosen’s breast pathology, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, pp. 187–229
Tan PH, Tse A, Lee A, Simpson JF, Hanby AM (2012) Fibroepithelial tumors. In: Lakhani SR, Ellis IO, Tan PH, van de Vijver MJ (eds) WHO classification of tumours of the breast, 4th edn. IARC Press, Lyon, pp. 142–147
Layfield LJ, Hart J, Neuwirth H, Bohman R, Trumbull WE, Giuliano AE (1989) Relation between DNA ploidy and the clinical behavior of phyllodes tumors. Cancer 64:1486–1489
Parker SJ, Harries SA (2001) Phyllodes tumours. Postgrad Med J 77:428–435
Ang MK, Ooi AS, Thike AA, Tan P, Zhang Z, Dykema K, Furge K, Teh BT, Tan PH (2011) Molecular classification of breast phyllodes tumors: validation of the histologic grading scheme and insights into malignant progression. Breast Cancer Res Treat 129:319–329
Esposito NN, Mohan D, Brufsky A, Lin Y, Kapali M, Dabbs DJ (2006) Phyllodes tumor: a clinicopathologic and immunohistochemical study of 30 cases. Arch Pathol Lab Med 130:1516–1521
Kersting C, Kuijper A, Schmidt H, Packeisen J, Liedtke C, Tidow N, Gustmann C, Hinrichs B, Wülfing P, Tio J, Boecker W, van Diest P, Brandt B, Buerger H (2006) Amplifications of the epidermal growth factor receptor gene (egfr) are common in phyllodes tumors of the breast and are associated with tumor progression. Lab Investig 86:54–61
Ho SK, Thike AA, Cheok PY, Tse GM, Tan PH (2013) Phyllodes tumours of the breast: the role of CD34, vascular endothelial growth factor and β-catenin in histological grading and clinical outcome. Histopathology 63:393–406
Kim SK, Jung WH, Koo JS (2014) Expression of Yes-associated protein (YAP) in breast phyllodes tumor. Int J Clin Exp Pathol 7:5997–6005
Tariq MU, Haroon S, Kayani N (2015) Role of CD10 immunohistochemical expression in predicting aggressive behavior of phylloides tumors. Asian Pac J Cancer Prev 16:3147–3152
Tawasil J, Go EM, Tsang JY, Ni YB, Ko CW, Tse GM (2015) Associations of epithelial c-kit expression in phyllodes tumours of the breast. J Clin Pathol 68:808–811
Bellezza G, Prosperi E, Del Sordo R, Colella R, Rulli A, Sidoni A (2016) IMP3 is strongly expressed in malignant phyllodes tumors of the breast: an immunohistochemical study. Int J Surg Pathol 24:37–42
Chambers AF, Matrisian LM (1997) Changing views of the role of matrix metalloproteinases in metastasis. Natl Cancer Inst 89:1260–1270
Vu T, Werb Z (2000) Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 14:2123–2133
Alford AI, Hankenson KD (2006) Matricellular proteins: extracellular modulators of bone development, remodeling, and regeneration. Bone 38:749–757
Framson PE, Sage EH (2004) SPARC and tumor growth: where the seed meets the soil? J Cell Biochem 92:679–690
Arnold SA, Brekken RA (2009) SPARC: a matricellular regulator of tumorigenesis. J Cell Commun Signal 3:255–273
Chlenski A, Cohn SL (2010) Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol 21:55–65
Barth PJ, Moll R, Ramaswamy A (2005) Stromal remodeling and SPARC (secreted protein acid rich in cysteine) expression in invasive ductal carcinomas of the breast. Virchows Arch 446:532–536
Hsiao YH, Lien HC, Hwa HL, Kuo WH, Chang KJ, Hsieh FJ (2010) SPARC (osteonectin) in breast tumors of different histologic types and its role in the outcome of invasive ductal carcinoma. Breast J 16:305–308
Ledda MF, Adris S, Bravo AI, Kairiyama C, Bover L, Chernajovsky Y, Mordoh J, Podhajcer OL (1997) Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med 3:171–176
Gilles C, Bassuk JA, Pulyaeva H, Sage EH, Foidart JM, Thompson EW (1998) SPARC/osteonectin induces matrix metalloproteinase 2 activation in human breast cancer cell lines. Cancer Res 58:5529–5536
Kunigal S, Gondi CS, Gujrati M, Lakka SS, Dinh DH, Olivero WC, Rao JS (2006) SPARC-induced migration of glioblastoma cell lines via uPA-uPAR signaling and activation of small GTPase RhoA. Int J Oncol 29:1349–1357
Kuroda N, Sugimoto T, Ueda S, Takahashi T, Moriki T, Sonobe H, Miyazaki E, Hayashi Y, Toi M, Hiroi M, Enzan H (2001) Malignant phyllodes tumor of the breast with expression of osteonectin and vinculin. Pathol Int 51:277–282
Jardim DL, Conley A, Subbiah V (2013) Comprehensive characterization of malignant phyllodes tumor by whole genomic and proteomic analysis: biological implications for targeted therapy opportunities. Orphanet J Rare Dis 8:112
Walther C, Hofvander J, Nilsson J, Magnusson L, Domanski HA, Gisselsson D, Tayebwa J, Doyle LA, Fletcher CD, Mertens F (2015) Gene fusion detection in formalin-fixed paraffin-embedded benign fibrous histiocytomas using fluorescence in situ hybridization and RNA sequencing. Lab Investig 95:1071–1076
Wang F, Flanagan J, Su N, Wang LC, Bui S, Nielson A, Wu X, Vo HT, Ma XJ, Luo Y (2012) RNAscope: a novel in situ RNA analysis platform for formalin-fixed, paraffin-embedded tissues. J Mol Diagn 14:22–29
Yuan J, Zhang J, Zhu Y, Li N, Tian T, Li Y, Li Y, Li Z, Lai Y, Gao J, Shen L (2016) Programmed death-ligand-1 expression in advanced gastric cancer detected with RNA in situ hybridization and its clinical significance. Oncotarget 7:39671–39679
Poenaru Sava MG, Raica ML, Cimpean AM (2014) VEGF mRNA assessment in human pterygium: a new ‘scope’ for a future hope. Ophthalmic Res 52:130–135
Shen LC, Chen YK, Hsue SS, Shaw SY (2010) Expression of osteonectin/secreted protein acidic and rich in cysteine and matrix metalloproteinases in ameloblastoma. J Oral Pathol Med 39:242–249
Zhao ZS, Wang YY, Chu YQ, Ye ZY, Tao HQ (2010) SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res 16:260–268
Duffy MJ, Maguire TM, ca, McDermott E, O’Higgins N (2000) Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res 2:252–257
Tremble PM, Lane TF, Sage EH, Werb Z (1993) SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol 121:1433–1444
Porte H, Triboulet JP, Kotelevets L, Carrat F, Prévot S, Nordlinger B, DiGioia Y, Wurtz A, Comoglio P, Gespach C, Chastre E (1998) Overexpression of stromelysin-3, BM-40/SPARC, and MET genes in human esophageal carcinoma: implications for prognosis. Clin Cancer Res 4:1375–1382
Acknowledgements
This manuscript was presented in part at the 10 European Breast Cancer Conference, Amsterdam, March 9–11, 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Institutional Review Board (IRB) at the Chonnam National University Hwasun Hospital (Jeollanam-do, Korea) (CNUHH-2014-155).
Funding
This study was supported by the Leading Foreign Research Institute Recruitment Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2011-0030034).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kim, N.I., Kim, GE., Lee, J.S. et al. In phyllodes tumors of the breast expression of SPARC (osteonectin/BM40) mRNA by in situ hybridization correlates with protein expression by immunohistochemistry and is associated with tumor progression. Virchows Arch 470, 91–98 (2017). https://doi.org/10.1007/s00428-016-2048-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-2048-0