Abstract
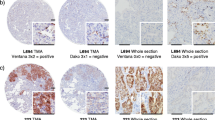
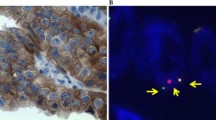
ALK gene rearrangements are identified in 2–5 % of all non-small cell lung cancer and are more common in lifetime non-smokers with adenocarcinoma, but the prevalence of ALK rearrangements is not as well characterized in long-term ex-smokers (quit >10 years prior to diagnosis). Accurate and timely diagnosis of ALK-rearranged tumors is of clinical importance given the remarkable response to targeted inhibitors. ALK gene rearrangement may be detected by fluorescence in situ hybridization (FISH), and abnormal expression of ALK protein may be detected by immunohistochemistry (IHC), the latter of which is faster and less expensive. The aim of this study is to evaluate the prevalence of ALK rearrangement in non-smokers and long-term ex-smokers with lung adenocarcinoma and to assess the performance of IHC for the detection of ALK+ tumors when compared to FISH. Two hundred fifty-one cases of resected lung adenocarcinoma were retrospectively reviewed, including non-smokers (n = 79) or long-term ex-smokers (n = 172). ALK IHC and ALK FISH were performed on each case. Four cases demonstrated ALK rearrangement by FISH (4/251; 1.6 %). All cases were non-smokers (4/79; 5.1 %), and all were positive for ALK by IHC. No additional cases were considered positive by IHC, and only 26 (10.4 %) cases were considered equivocal using a conservative approach to interpretation, resulting in a sensitivity of 100 % and specificity of 89.5 %. ALK rearrangement was not observed in lung adenocarcinoma arising in long-term ex-smokers, whereas it is seen in up to 5.1 % of lifetime non-smokers. ALK IHC using the 5A4 antibody demonstrates high sensitivity, supporting its use as a screening test.

Similar content being viewed by others
References
Islami F, Torre L, Jemal A (2015) Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 4(4):327–338
Tobin NP, Foukakis T, De Petris L, et al. (2015) The importance of molecular markers for diagnosis and selection of targeted treatments in patients with cancer. J Intern Med 278(6):545–570. doi:10.1111/joim.12429
Subramanian J, Govindan R (2007) Lung cancer in never smokers: a review. J Clin Oncol 25(5):561–570
Sampsonas F, Ryan D, McPhillips D, et al. (2014) Molecular testing and personalized treatment of lung cancer. Curr Mol Pharmacol 7(1):22–32
Popper H, Ryska A, Tímár J, et al. (2014) Molecular testing in lung cancer in the era of precision medicine. Transl Lung Cancer Res 3(5):291–300
Ali S, Ignatius Ou S, He J, et al. (2014) Identifying ALK rearrangements that are not detected by FISH with targeted next generation sequencing of lung carcinoma. J Clin Oncol 32(5s):suppl; abstr 8049.
Han X, Zhang N, Ma L, et al. (2013) Immunohistochemistry reliably detects ALK rearrangements in patients with advanced non-small-cell lung cancer. Virchows Arch 463(4):583–591
Iyevleva A, Raskin G, Tiurin V, et al. (2015) Novel ALK fusion partners in lung cancer. Cancer Lett 362(1):116–121
Wallander M, Geiersbach K, Tripp S, et al. (2012) Comparison of reverse transcription-polymerase chain reaction, immunohistochemistry, and fluorescence in situ hybridization methodologies for detection of echinoderm microtubule-associated proteinlike 4-anaplastic lymphoma kinase fusion-positive non-small cell lung carcinoma: implications for optimal clinical testing. Arch Pathol Lab Med 136(7):796–803
Conklin CMJ, Craddock K, Have C, et al. (2013) Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol 8(1):45–51
Approval letter RE: P140025 (VENTANA ALK (D5F3) CDx Assay) [Internet].: Food and Drug Administration; 2015 [updated 07/10/2015; cited 12/13/2015]. Available from: http://www.accessdata.fda.gov/cdrh_docs/pdf14/P140025a.pdf.
Doshi S, Ray D, Stein K, et al. (2016) Economic analysis of alternative strategies for detection of ALK rearrangements in non small cell lung cancer. Diagnostics (Basel) 6(1). doi: 10.3390/diagnostics6010004
Sun J, Choi Y, Won J, et al. (2012) A dramatic response to crizotinib in a non-small-cell lung cancer patient with IHC-positive and FISH-negative ALK. J Thorac Oncol 7(12):e36–e38
Ren S, Hirsch F, Varella Garcia M, et al. (2014) Atypical negative ALK break-apart FISH harboring a crizotinib-responsive ALK rearrangement in non-small-cell lung cancer. J Thorac Oncol 9(3):e21–e23
Peled N, Palmer G, Hirsch F, et al. (2012) Next-generation sequencing identifies and immunohistochemistry confirms a novel crizotinib-sensitive ALK rearrangement in a patient with metastatic non-small-cell lung cancer. J Thorac Oncol 7(9):e14–e16
Sholl L, Weremowicz S, Gray S, et al. (2013) Combined use of ALK immunohistochemistry and FISH for optimal detection of ALK-rearranged lung adenocarcinomas. J Thorac Oncol 8(3):322–328
Cutz J, Craddock K, Torlakovic E, et al. (2014) Canadian anaplastic lymphoma kinase study: a model for multicenter standardization and optimization of ALK testing in lung cancer. J Thorac Oncol 9(9):1255–1263
Pekar Zlotin M, Hirsch F, Soussan Gutman L, et al. (2015) Fluorescence in situ hybridization, immunohistochemistry, and next-generation sequencing for detection of EML4-ALK rearrangement in lung cancer. Oncologist 20(3):316–322
Hagemann I, Devarakonda S, Lockwood C, et al. (2015) Clinical next-generation sequencing in patients with non-small cell lung cancer. Cancer 121(4):631–639
Pritchard C, Salipante S, Koehler K, et al. (2014) Validation and implementation of targeted capture and sequencing for the detection of actionable mutation, copy number variation, and gene rearrangement in clinical cancer specimens. J Mol Diagn. 16(1):56–67
Shackelford R, Vora M, Mayhall K, et al. (2014) ALK-rearrangements and testing methods in non-small cell lung cancer: a review. Genes Cancer 5(1–2):1–14
Rodig S, Mino Kenudson M, Dacic S, et al. (2009) Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 15(16):5216–5223
Greer W, Douglas S, Xu Z, et al. (2015) Molecular diagnostics for lung cancer in Atlantic Canada. Canadian Journal of Pathology 7(1):20–26
Lindeman N, Cagle P, Beasley M, et al. (2013) Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Mol Diagn. 15(4):415–453
Yi E, Boland J, Maleszewski J, et al. (2011) Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 6(3):459–465
Minca E, Portier B, Wang Z, et al. (2013) ALK status testing in non-small cell lung carcinoma: correlation between ultrasensitive IHC and FISH. J Mol Diagn 15(3):341–346
Cabillic F, Gros A, Dugay F, et al. (2014) Parallel FISH and immunohistochemical studies of ALK status in 3244 non-small-cell lung cancers reveal major discordances. J Thorac Oncol 9(3):295–306
Ilie M, Hofman P (2015) Reply to the letter to the editor ‘ALK FISH rearranged and amplified tumor with negative immunohistochemistry: a rare and challenging case concerning ALK status screening in lung cancer’ by Uguen et al. Ann Oncol 26(8):1802
Djalalov S, Beca J, Hoch J, et al. (2014) Cost effectiveness of EML4-ALK fusion testing and first-line crizotinib treatment for patients with advanced ALK-positive non-small-cell lung cancer. J Clin Oncol 32(10):1012–1019. doi:10.1200/JCO.2013.53.1186
Kerr KM, Bubendorf L, Edelman MJ, et al. (2014) Second ESMO consensus conference on lung cancer: pathology and molecular biomarkers for non-small-cell lung cancer. Ann Oncol 25(9):1681–1690. doi:10.1093/annonc/mdu145
Acknowledgments
The authors would like to acknowledge the excellent technical support received from Marissa Goudie, Ossayed Alawor, Makoto Matsueka, and Alison MacDonald and clerical support from Joy Douglas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study adhered to scientific and medical standards of ethics and was approved by the regional Research Ethics Board (CDHA-RS/2012-029).
Funding
This study was funded by an Investigator Initiated Research grant from Pfizer (#WS1416261).
Conflict of interest
Dr. Zhaolin Xu is a current member of Pfizer’s National Lung Cancer Medical Advisory Board. Dr. Drew Bethune has been a member of Pfizer’s Medical Advisory Board and AstraZeneca’s Medical Advisory Board. None of the remaining authors declare any potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Williams, A.S., Greer, W., Bethune, D. et al. ALK+ lung adenocarcinoma in never smokers and long-term ex-smokers: prevalence and detection by immunohistochemistry and fluorescence in situ hybridization. Virchows Arch 469, 533–540 (2016). https://doi.org/10.1007/s00428-016-2005-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-2005-y