Abstract
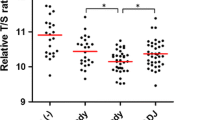
Telomere shortening occurs in many organs and tissues and is accelerated by oxidative injury and rapid cell turnover. Short telomeres initiate chromosomal instability and may eventually contribute to tumorigenesis. To evaluate telomere length as potential biomarker for gastric cancer (GC) risk, we measured average telomere length using quantitative real-time PCR in GC tissues and in non-neoplastic mucosa from patients with GC and without GC. We obtained of 217 GC patients matched biopsies from the GC and adjacent tissues as well as gastric biopsies of 102 subjects without GC. Relative telomere length was measured in genomic DNA by real-time PCR. Relative telomere length decreased gradually in Helicobacter pylori (H. pylori) negative and positive gastric mucosa of GC free subjects compared with adjacent mucosa and cancer tissue from GC patients (4.03 ± 0.3 vs. 2.82 ± 0.19 vs. 0.82 ± 0.07 vs. 0.29 ± 0.09, P < 0.0001). In non-neoplastic mucosa of GC patients, shorter telomeres were found significantly more often than in that of GC free subjects (age, sex, and H. pylori adjusted odds ratio = 7.81, 95 % confidence interval = 4.71–12.9, P < 0.0001). Telomere shortening in non-neoplastic mucosa was associated with chronic inflammation (P = 0.0018) and intestinal metaplasia (P < 0.0001). No significant associations were found between relative telomere length and clinicopathological features of GC and overall survival. Telomere shortening in gastric mucosa reflects a field effect in an early stage of carcinogenesis and is associated with an increased risk of GC. Telomere length in GC is not associated with clinicopathological features or prognosis.



Similar content being viewed by others
References
Ferlay J, Bray F, Parkin DM, Pisani P (2001) Gobocan 2000: cancer prevalence and mortality worldwide (IARC cancer bases no. 5). IARC Press, Lyon
Lau M, Le A, El-Serag HB (2006) Noncardia gastric adenocarcinoma remains an important and deadly cancer in the United States: secular trends in prevalence and survival. Am J Gastroenterol 101:2485–2492
Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. (2013) Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer 16:1–27
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345:784–789
de Lange T (2005) The protein complex that shapes and safeguards human telomeres. Genes Dev 19:2100–2110
von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27:339–344
Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, et al. (2003) Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst 95:1211–1218
Harley CB, Futcher AB, Greider CW (1990) Telomeres shorten during ageing of human fibroblasts. Nature 345:458–460
Vulliamy T, Marrone A, Dokal I, Mason PJ (2002) Association between aplastic anaemia and mutations in telomerase RNA. Lancet 359:2168–2070
Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6:611–622
Chang S (2005) Modeling aging and cancer in the telomerase knockout mouse. Mutat Res 576:39–53
Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, et al. (2000) Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406:641–645
Bailey SM, Murnane JP (2006) Telomeres, chromosome instability and cancer. Nucleic Acids Res 34:2408–2017
Cheung AL, Deng W (2008) Telomere dysfunction, genome instability and cancer. Front Biosci 13:2075–2090
Murnane JP (2006) Telomeres and chromosome instability. DNA Repair (Amst) 5:1082–1092
Parsonnet J (1993) Helicobacter pylori and gastric cancer. Gastroenterol Clin N Am 22:89–104
Aida J, Izumiyama-Shimomura N, Nakamura K, Ishii A, Ishikawa N, Honma N, et al. (2007) Telomere length variations in 6 mucosal cell types of gastric tissue observed using a novel quantitative fluorescence in situ hybridization method. Hum Pathol 38:1192–1200
Tahara T, Shibata T, Kawamura T, Ishizuka T, Okubo M, Nagasaka M, Nakagawa Y, Arisawa T, Ohmiya N, Hirata I (2016) Telomere length in non-neoplastic gastric mucosa and its relationship to H. pylori infection, degree of gastritis, and NSAID use. Clin Exp Med. 16:65–71
Maruyama Y, Hanai H, Fujita M, Kaneko E (1997) Telomere length and telomerase activity in carcinogenesis of the stomach. Jpn J Clin Oncol 27:216–220
Mu Y, Zhang Q, Mei L, Liu X, Yang W, Yu J (2012) Telomere shortening occurs early during gastrocarcinogenesis. Med Oncol 29:893–898
Cawthon RM (2002) Telomere measurement by quantitative PCR. Nucleic Acids Res 30:e47
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 64:31–49
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis: the updated Sydney system. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 20:1161–1181
McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I (2007) Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomark Prev 16:815–819
Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, et al. (1997) Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomark Prev 6:639–642
Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. (2004) China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291:187–194
Hosokawa O, Tsuda S, Kidani E, Watanabe K, Tanigawa Y, Shirasaki S, et al. (1998) Diagnosis of gastric cancer up to three years after negative upper gastrointestinal endoscopy. Endoscopy 30:721–773
Aslan R, Bektas A, Bedir A, Alacam H, Aslan MS, Nar R, et al. (2013) Helicobacter pylori eradication increases telomere length in gastric mucosa. Hepato-Gastroenterology 60:601–604
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 54 kb)
Rights and permissions
About this article
Cite this article
Tahara, T., Shibata, T., Kawamura, T. et al. Telomere length shortening in gastric mucosa is a field effect associated with increased risk of gastric cancer. Virchows Arch 469, 19–24 (2016). https://doi.org/10.1007/s00428-016-1948-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-1948-3