Abstract
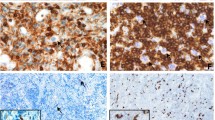
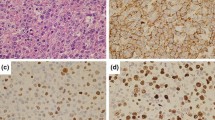
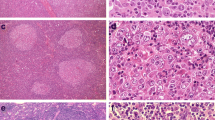
Although the vast majority of diffuse large B-cell lymphomas (DLBCL) are negative for the Epstein-Barr virus (EBV), a subset of DLBCL in immunocompetent patients carries EBV in the lymphoma cells and expresses EBV-encoded RNA and/or proteins. EBV-positive DLBCL are rare and represent either pyothorax associated DLBCL or EBV-positive DLBCL of the elderly. In all cases of EBV-positive DLBCL, EBV is detectable in virtually all lymphoma cells, indicating that EBV was present at an initial phase of lymphomagenesis. We identified four lymphomas with an unusual EBV expression pattern. The majority of B-cell blasts were EBV-negative, but accompanied by a small number (less than 10 % of all B-cells) of EBV-positive B-cells with blastic morphology. The median age of the patients was 56.5 years, and no clinically evident immunosuppression was reported. In one patient EBV-positive blasts occurred in the gastric mucosa which resembled an EBV-positive mucocutaneous ulcer. In all cases EBV-positive B-cells occurred in small loose clusters within or adjacent to an EBV-negative DLBCL. In one case we were able to study immunoglobulin gene rearrangement in microdissected EBV-positive B-cells and the EBV-negative lymphoma compartment. This revealed different rearrangement patterns in EBV-negative DLBCL than in EBV-positive peritumoral B-cells, without clonal relationship between these B-cell populations. Despite the molecular data being limited to one patient, we suggest that in close proximity to EBV-negative DLBCL intra- and peritumoral EBV-positive B-cells may occur, possibly due to local immune escape mechanisms. This represents a diagnostic pitfall.



Similar content being viewed by others
References
Armitage JO, Weisenburger DD (1998) New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major Histologic subtypes. Non-Hodgkin’s lymphoma classification project. J Clin Oncol: Off J Am Soc Clin Oncol 8:2780–2795
Swerdlow SH (2008) WHO classification of tumours of haematopoietic and lymphoid tissues. Internat. Agency for Research on Cancer, Lyon
Menon MP, Hutchinson L, Garver J, Jaffe ES, Woda BA (2013) Transformation of follicular lymphoma to Epstein-Barr virus-related Hodgkin-like lymphoma. J Clin Oncol: Off J Am Soc Clin Oncol 5:e53–6
Petitjean B, Jardin F, Joly B, Martin-Garcia N, Tilly H, Picquenot J-M, Briere J, Danel C, Mehaut S, Abd-Al-Samad I, Copie-Bergman C, Delfau-Larue M-H, Gaulard P (2002) Pyothorax-associated lymphoma: a peculiar clinicopathologic entity derived from B cells at late stage of differentiation and with occasional aberrant dual B- and T-cell phenotype. Am J Surg Pathol 6:724–732
Fukayama M, Ibuka T, Hayashi Y, Ooba T, Koike M, Mizutani S (1993) Epstein-Barr virus in pyothorax-associated pleural lymphoma. Am J Pathol 4:1044–1049
Oyama T, Yamamoto K, Asano N, Oshiro A, Suzuki R, Kagami Y, Morishima Y, Takeuchi K, Izumo T, Mori S, Ohshima K, Suzumiya J, Nakamura N, Abe M, Ichimura K, Sato Y, Yoshino T, Naoe T, Shimoyama Y, Kamiya Y, Kinoshita T, Nakamura S (2007) Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res: Off J Am Assoc Cancer Res 17:5124–5132
Montes-Moreno S, Odqvist L, Diaz-Perez JA, Lopez AB, de Villambrosía SG, Mazorra F, Castillo ME, Lopez M, Pajares R, García JF, Mollejo M, Camacho FI, Ruiz-Marcellán C, Adrados M, Ortiz N, Franco R, Ortiz-Hidalgo C, Suarez-Gauthier A, Young KH, Piris MA (2012) EBV-positive diffuse large B-cell lymphoma of the elderly is an aggressive post-germinal center B-cell neoplasm characterized by prominent nuclear factor-kB activation. Mod Pathol: Off J U S Can Acad Pathol, Inc 7:968–982
Oyama T, Ichimura K, Suzuki R, Suzumiya J, Ohshima K, Yatabe Y, Yokoi T, Kojima M, Kamiya Y, Taji H, Kagami Y, Ogura M, Saito H, Morishima Y, Nakamura S (2003) Senile EBV + B-cell lymphoproliferative disorders: a clinicopathologic study of 22 patients. Am J Surg Pathol 1:16–26
Shimoyama Y, Yamamoto K, Asano N, Oyama T, Kinoshita T, Nakamura S (2008) Age-related Epstein-Barr virus-associated B-cell lymphoproliferative disorders: special references to lymphomas surrounding this newly recognized clinicopathologic disease. Cancer Sci 6:1085–1091
Nakatsuka S-I, Yao M, Hoshida Y, Yamamoto S, Iuchi K, Aozasa K (2002) Pyothorax-associated lymphoma: a review of 106 cases. J Clin Oncol: Off J Am Soc Clin Oncol 20:4255–4260
Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES (2010) EBV positive mucocutaneous ulcer–a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol 3:405–417
Dojcinov SD, Venkataraman G, Pittaluga S, Wlodarska I, Schrager JA, Raffeld M, Hills RK, Jaffe ES (2011) Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood 18:4726–4735
Thorley-Lawson DA, Gross A (2004) Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 13:1328–1337
Thomas JA, Hotchin NA, Allday MJ, Amlot P, Rose M, Yacoub M, Crawford DH (1990) Immunohistology of Epstein-Barr virus-associated antigens in B cell disorders from immunocompromised individuals. Transplantation 5:944–953
Shimoyama Y, Oyama T, Asano N, Oshiro A, Suzuki R, Kagami Y, Morishima Y, Nakamura S (2006) Senile Epstein-Barr virus-associated B-cell lymphoproliferative disorders: a mini review. J Clin Exp Hematopathol: JCEH 1:1–4
van Dongen JJM, Langerak AW, Brüggemann M, Evans PAS, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, García-Sanz R, van Krieken JHJM, Droese J, González D, Bastard C, White HE, Spaargaren M, González M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA (2003) Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 12:2257–2317
Nakayama S, Yokote T, Iwaki K, Hirata Y, Nishiwaki U, Akioka T, Miyoshi T, Masuda Y, Tsuji M, Hanafusa T (2012) Multiple cytokine- and chemokine-producing primary testicular diffuse large B-cell lymphoma, not otherwise specified. Leuk Res 8:e171–4
Knowles DM (1999) Immunodeficiency-associated lymphoproliferative disorders. Mod Pathol: Off J U S Can Acad Pathol, Inc 2:200–217
Langerak AW, Groenen PJTA, Bruggemann M, Beldjord K, Bellan C, Bonello L, Boone E, Carter GI, Catherwood M, Davi F, Delfau-Larue M-H, Diss T, Evans PAS, Gameiro P, Garcia Sanz R, Gonzalez D, Grand D, Hakansson A, Hummel M, Liu H, Lombardia L, Macintyre EA, Milner BJ, Montes-Moreno S, Schuuring E, Spaargaren M, Hodges E, van Dongen JJM (2012) EuroClonality/BIOMED-2 guidelines for interpretation and reporting of Ig/TCR clonality testing in suspected lymphoproliferations. Leukemia 10:2159–2171
Park S, Lee J, Ko YH, Han A, Jun HJ, Lee SC, Hwang IG, Park YH, Ahn JS, Jung CW, Kim K, Ahn YC, Kang WK, Park K, Kim WS (2007) The impact of Epstein-Barr virus status on clinical outcome in diffuse large B-cell lymphoma. Blood 3:972–978
Hoeller S, Tzankov A, Pileri SA, Went P, Dirnhofer S (2010) Epstein-Barr virus-positive diffuse large B-cell lymphoma in elderly patients is rare in Western populations. Hum Pathol 3:352–357
Gibson SE, Hsi ED (2009) Epstein-Barr virus-positive B-cell lymphoma of the elderly at a United States tertiary medical center: an uncommon aggressive lymphoma with a nongerminal center B-cell phenotype. Hum Pathol 5:653–661
Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Muller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC (2004) Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 1:275–282
Nerurkar AY, Vijayan P, Srinivas V, Soman CS, Dinshaw KA, Advani SH, Magrath I, Bhatia K, Naresh KN (2000) Discrepancies in Epstein-Barr virus association at presentation and relapse of classical Hodgkin’s disease: impact on pathogenesis. Ann Oncol: Off J Eur Soc Med Oncol / ESMO 4:475–478
Shimizu N, Tanabe-Tochikura A, Kuroiwa Y, Takada K (1994) Isolation of Epstein-Barr virus (EBV)-negative cell clones from the EBV-positive Burkitt’s lymphoma (BL) line Akata: malignant phenotypes of BL cells are dependent on EBV. J Virol 9:6069–6073
Acknowledgements
The authors would like to thank Charlotte Botz-von Drathen and Olivera Batic for their excellent technical assistance. The work was supported by the Dr. Werner Jackstädt-Stiftung Junior Excellence Research Group “Mechanisms of B-cell lymphoma development at old age as a basis for age adjusted therapeutic strategies“. We are indebted to the treating physicians and referring pathologists.
Disclosure
The work was supported by the Dr. Werner Jackstädt-Stiftung Junior Excellence Research Group “Mechanisms of B-cell lymphoma development at old age as a basis for age adjusted therapeutic strategies“. The authors have no conflict of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Christiane Stuhlmann-Laeisz and Monika Szczepanowski these authors contribute equally
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary figure 1
Comparative Immunoglobulin rearrangement analysis of EBV-negative DLBCL and the microdissected EBV-positive cells (case 1, Table 1). Fragment analysis of microdissected EBV-positive B-cells and EBV-negative DLBCL. Amplifying primers are indicated above the electropherograms; the analyzed probes are indicated on the left. A: PCR using BIOMED primer IgH DH (1–6) JH. No detectable clonal amplicon in the microdissected EBV-positive cells, whereas there is a clonal amplicon of 405 bp present in the EBV-negative DLBCL. B PCR using BIOMED primer IgL (Vλ-Jλ). No detectable clonal amplicon in the microdissected EBV-positive B-cells, whereas there is a clonal amplicon of 151 bp present in the EBV-negative DLBCL. (TIFF 8513 kb)
Supplementary figure 2
Laser-assisted microdissection (case 1, Table 1). Example of microdissected EBV-positive cells (A). Using laser cut (B). EBV-positive single cells were separated, stuck onto the tube lid and lifted up (C). Original magnification, x400 (TIFF 6243 kb)
Supplementary table 1
(DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Stuhlmann-Laeisz, C., Szczepanowski, M., Borchert, A. et al. Epstein-Barr virus-negative diffuse large B-cell lymphoma hosts intra- and peritumoral B-cells with activated Epstein-Barr virus. Virchows Arch 466, 85–92 (2015). https://doi.org/10.1007/s00428-014-1661-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1661-z