Abstract
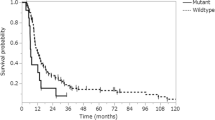
We investigated the significance of PI3K/AKT/mTOR pathway and its interactions with MAPK, JAK/STAT and Notch pathways in meningioma progression. Paraffin-embedded tissue from 108 meningioma patients was analysed for the presence of mutations in PIK3CA and AKT1. These were correlated with the expression status of components of the PI3K/AKT/mTOR pathway, including p85α and p110γ subunits of PI3K, phosphorylated (p)-AKT, p-mTOR, p-p70S6K and p-4E-BP1, as well as of p-ERK1/2, p-STAT3 and Notch-1, clinicopathological data and patient survival. A mutation in PIK3CA or AKT1 was found in around 9 % of the cases. Higher grade meningiomas displayed higher nuclear expression of p-p70S6K; higher nuclear and cytoplasmic expression of p-4E-BP1 and of Notch-1; lower cytoplasmic expression of p85αPI3K, p-p70S6K and p-ERK1/2; and lower PTEN Histo-scores (H-scores). PTEN H-score was inversely correlated with recurrence probability. In univariate survival analysis, nuclear expression of p-4E-BP1 and absence of p-ERK1/2 expression portended adverse prognosis, whereas in multivariate survival analysis, p-ERK1/2 expression emerged as an independent favourable prognostic factor. Treatment of the human meningioma cell line HBL-52 with the PI3K inhibitor LY294002 resulted in reduction of p-AKT, p-p70S6K and p-ERK1/2 protein levels. The complex interactions established between components of the PI3K/AKT/mTOR pathway, or with components of the MAPK, JAK/STAT and Notch-1 pathways, appear to be essential for facilitating and fuelling meningioma progression.






Similar content being viewed by others
References
Pham MH et al (2011) Molecular genetics of meningiomas: a systematic review of the current literature and potential basis for future treatment paradigms. Neurosurg Focus 30:E7
Riemenschneider MJ, Perry A, Reifenberger G (2006) Histological classification and molecular genetics of meningiomas. Lancet Neurol 5:1045–1054
Jaskolski DJ et al (2013) Molecular alterations in meningiomas: association with clinical data. Clin Neuropathol 32:114–121
Barbera S et al (2013) Genetic changes with prognostic value in histologically benign meningiomas. Clin Neuropathol 32:311–317
Yuan TL, Cantley LC (2008) PI3K pathway alterations in cancer: variations on a theme. Oncogene 27:5497–5510
De Luca A, Maiello MR, D’Alessio A, Pergameno M, Normanno N (2012) The RAS/RAF/MEK/ERK and the PI3K/AKT signalling pathways: role in cancer pathogenesis and implications for therapeutic approaches. Expert Opin Ther Targets 16:S17–S27
Logue JS, Morrison DK (2012) Complexity in the signaling network: insights from the use of targeted inhibitors in cancer therapy. Genes Dev 26:641–650
Vogt PK, Hart JR (2011) PI3K and STAT3: a new alliance. Cancer Discov 1:481–486
Gutierrez A, Look AT (2007) NOTCH and PI3K-AKT pathways intertwined. Cancer Cell 12:411–413
Wong GW, Knowles GC, Mak TW, Ferrando AA, Zúñiga-Pflücker JC (2012) HES1 opposes a PTEN-dependent check on survival, differentiation, and proliferation of TCRβ-selected mouse thymocytes. Blood 120:1439–1448
Gymnopoulos M, Elsliger MA, Vogt PK (2007) Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci U S A 104:5569–5574
Steelman LS et al (2011) Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy—implications for cancer and aging. Aging (Albany NY) 3:192–222
Carpten JD et al (2007) A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448:439–444
Chetram MA, Hinton CV (2012) PTEN regulation of ERK1/2 signaling in cancer. J Recept Signal Transduct Res 32:190–195
Korkolopoulou P et al (2012) A comprehensive immunohistochemical and molecular approach to the PI3K/AKT/mTOR (phosphoinositide 3-kinase/v-akt murine thymoma viral oncogene/mammalian target of rapamycin) pathway in bladder urothelial carcinoma. BJU Int 110:E1237–E1248
Rojo F et al (2007) 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res 13:81–89
Mair R, Morris K, Scott I, Carroll TA (2011) Radiotherapy for atypical meningiomas. J Neurosurg 115:811–819
Vranic A, Peyre M, Kalamarides M (2012) New insights into meningioma: from genetics to trials. Curr Opin Oncol 24:660–665
Pang JC et al (2006) Rare mutation of PIK3CA in meningiomas. Acta Neuropathol 111:284–285
Eom HS, Kim MS, Hur SY, Yoo NJ, Lee SH (2009) Absence of oncogenic AKT1 E17K mutation in prostate, esophageal, laryngeal and urothelial carcinomas, hepatoblastomas, gastrointestinal stromal tumors and malignant meningiomas. Acta Oncol 48:1084–1085
Brastianos PK et al (2013) Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet 45:285–289
Clark VE et al (2013) Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339:1077–1080
Juanpere N et al (2012) Mutations in FGFR3 and PIK3CA, singly or combined with RAS and AKT1, are associated with AKT but not with MAPK pathway activation in urothelial bladder cancer. Hum Pathol 43:1573–1582
McCubrey JA et al (2012) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget 3:1068–1111
Konstantinidou AE et al (2003) Hormone receptors in non-malignant meningiomas correlate with apoptosis, cell proliferation and recurrence-free survival. Histopathology 43:280–290
Beckner ME et al (2013) Low-level amplification of oncogenes correlates inversely with age for patients with nontypical meningiomas. World Neurosurg 79:313–319
Johnson MD, O’Connell MJ, Pilcher W, Reeder JE (2010) Fibroblast growth factor receptor-3 expression in meningiomas with stimulation of proliferation by the phosphoinositide 3 kinase-Akt pathway. J Neurosurg 112:934–939
Johnson MD, Okedli E, Woodard A, Toms SA, Allen GS (2002) Evidence for phosphatidylinositol 3-kinase-Akt-p7S6K pathway activation and transduction of mitogenic signals by platelet-derived growth factor in meningioma cells. J Neurosurg 97:668–675
Roberts PJ, Der CJ (2007) Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 26:3291–3310
Xu Q et al (2005) Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene 24:5552–5560
Johnson MD, O’Connell M, Vito F, Bakos RS (2009) Increased STAT3 and synchronous activation of Raf-1-MEK-1-MAPK, and phosphatidylinositol 3-Kinase-Akt-mTOR pathways in atypical and anaplastic meningiomas. J Neurooncol 92:129–136
Le Page C, Koumakpayi IH, Alam-Fahmy M, Mes-Masson AM, Saad F (2006) Expression and localisation of Akt-1, Akt-2 and Akt-3 correlate with clinical outcome of prostate cancer patients. Br J Cancer 94:1906–1912
Badve S et al (2010) Subcellular localization of activated AKT in estrogen receptor- and progesterone receptor-expressing breast cancers: potential clinical implications. Am J Pathol 176:2139–2149
Dobashi Y et al (2009) Critical and diverse involvement of Akt/mammalian target of rapamycin signaling in human lung carcinomas. Cancer 115:107–118
Johnson SM et al (2010) Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J Am Coll Surg 210:767–778
Levidou G et al (2013) A comprehensive immunohistochemical approach of AKT/mTOR pathway and p-STAT3 in mycosis fungoides. J Am Acad Dermatol 69:375–384
Zhao N, Guo Y, Zhang M, Lin L (2010) AKT-mTOR signalling is involved in Notch-1-mediated gliomas cell survival and proliferation. Oncol Rep 23:1443–1447
Zhou J et al (2007) Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A 104:16158–16163
Pachow D et al (2013) mTORC1 inhibitors suppress meningioma growth in mouse models. Clin Cancer Res 19:1180–1189
Riemenschneider MJ, Betensky RA, Pasedag SM, Louis DN (2006) AKT activation in human glioblastomas enhances proliferation via TSC2 and S6 kinase signaling. Cancer Res 66:5618–5623
Ge W, Ren J (2012) mTOR-STAT3-notch signalling contributes to ALDH2-induced protection against cardiac contractile dysfunction and autophagy under alcoholism. J Cell Mol Med 16:616–626
Pende M et al (2004) S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol 24:3112–3124
Cohen JD et al (2011) ERK crosstalks with 4EBP1 to activate cyclin D1 translation during quinol-thioether-induced tuberous sclerosis renal cell carcinoma. Toxicol Sci 124:75–87
Rolli-Derkinderen M et al (2003) ERK and p38 inhibit the expression of 4E-BP1 repressor of translation through induction of Egr-1. J Biol Chem 278:18859–18867
Korkolopoulou P et al (2012) Phosphorylated 4E-binding protein 1 (p-4E-BP1): a novel prognostic marker in human astrocytomas. Histopathology 61:293–305
Xiao L, Wang YC, Li WS, Du Y (2009) The role of mTOR and phospho-p70S6K in pathogenesis and progression of gastric carcinomas: an immunohistochemical study on tissue microarray. J Exp Clin Cancer Res 28:152
Mawrin C et al (2005) Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res 11:4074–4082
Jiang L et al (2011) Notch 1 expression is upregulated in glioma and is associated with tumor progression. J Clin Neurosci 18:387–390
Chung JH, Eng C (2005) Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res 65:8096–8100
Gimm O et al (2000) Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol 156:1693–1700
Song MS, Salmena L, Pandolfi PP (2012) The functions and regulation of the PTEN tumor suppressor. Nat Rev Mol Cell Biol 13:283–296
Choucair K et al (2012) PTEN genomic deletion predicts prostate cancer recurrence and is associated with low AR expression and transcriptional activity. BMC Cancer 12:543
McMenamin ME et al (1999) Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res 59:4291–4296
Mueller S et al (2012) PTEN promoter methylation and activation of the PI3K/Akt/mTOR pathway in pediatric gliomas and influence on clinical outcome. Neuro Oncol 14:1146–1152
Castellvi J et al (2006) Phosphorylated 4E binding protein 1: a hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer 107:1801–1811
Adeyinka A et al (2002) Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res 8:1747–1753
Chadha KS et al (2006) Activated Akt and Erk expression and survival after surgery in pancreatic carcinoma. Ann Surg Oncol 13:933–939
Givant-Horwitz V et al (2003) Mitogen-activated protein kinases (MAPK) as predictors of clinical outcome in serous ovarian carcinoma in effusions. Gynecol Oncol 91:160–172
Milde-Langosch K et al (2005) Expression and prognostic relevance of activated extracellular-regulated kinases (ERK1/2) in breast cancer. Br J Cancer 92:2206–2215
Mizumoto Y et al (2007) Activation of ERK1/2 occurs independently of KRAS or BRAF status in endometrial cancer and is associated with favorable prognosis. Cancer Sci 98:652–658
Schmitz KJ et al (2007) Activation of extracellular regulated kinases (ERK1/2) but not AKT predicts poor prognosis in colorectal carcinoma and is associated with k-ras mutations. Virchows Arch 450:151–159
Vicent S et al (2004) ERK1/2 is activated in non-small-cell lung cancer and associated with advanced tumors. Br J Cancer 90:1047–1052
Saetta AA et al (2011) Expression of pERK and pAKT in human astrocytomas: correlation with IDH1-R132H presence, vascular endothelial growth factor, microvascular characteristics and clinical outcome. Virchows Arch 458:749–759
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Elias A. El-Habr, Georgia Levidou, Eleni-Andriana Trigka and Joanna Sakalidou have equally contributed to this manuscript.
Angelica A. Saetta and Penelope Korkolopoulou are senior authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 60 kb)
Supplementary Table 2
(DOC 67 kb)
Supplementary Table 3
(DOC 106 kb)
Supplementary Table 4
(DOC 189 kb)
Fig. S1
p-AKT, p-p70S6K, and p-ERK1/2 protein levels in fresh frozen tissue specimens by Western blot and immunohistochemical expression of p-AKT, p-p70S6K and p-ERK1/2 in formalin-fixed tissue from the same five specimens. (A) Detection of p-AKT in the five cases. Tumours #2, 5 showed the highest p-AKT levels in frozen and paraffin-embedded samples. (B) Detection of p-p70S6K in the five cases. Tumours #1, 3, 5 showed the highest p-p70S6K levels in frozen and paraffin-embedded samples. (C) Detection of p-ERK1/2 in the five cases. Tumours #1, 2, 5 showed the highest p-ERK1/2 levels in frozen and paraffin-embedded samples (GIF 257 kb)
Fig. S2
Detection of p-AKT, p-p70S6K and p-ERK1/2 protein levels in the meningioma cell line HBL-52 before and after 3 h treatment with PI3K inhibitor LY294002 (100 μM). Actin was used as an internal control for Western blot analysis in all experiment (GIF 24 kb)
Rights and permissions
About this article
Cite this article
El-Habr, E.A., Levidou, G., Trigka, EA. et al. Complex interactions between the components of the PI3K/AKT/mTOR pathway, and with components of MAPK, JAK/STAT and Notch-1 pathways, indicate their involvement in meningioma development. Virchows Arch 465, 473–485 (2014). https://doi.org/10.1007/s00428-014-1641-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1641-3