Abstract
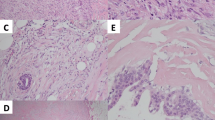
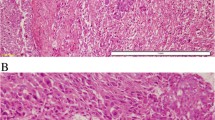
Fibromatosis-like metaplastic carcinoma (FLMCa) of the breast is a rare low-grade spindle cell carcinoma, of which the biological characteristics have not been well studied. This study aims to assess, in FLMCa, immunohistochemical expression of claudins (CLDN) and features connected with the claudin-low subtype, such as the presence of tumor initiating cells (TIC), epithelial–mesenchymal transition (EMT) phenotype, as well as EGFR activating mutations. Three cases of FLMCa were retrieved from our hospital archives. Histological and immunohistochemical characteristics were reviewed. Expression of CLDN-1, CLDN-3, CLDN-4 and CLDN-7, CD44 and CD24 (TIC phenotype), and vimentin and E-cadherin (EMT features) were studied. EGFR mutations on exons 18, 19, 20, and 21 were investigated by real-time PCR. In all cases, the low-grade spindle cell component was predominant, with two cases presenting <5 % of epithelioid and squamous areas. The tumors expressed basal cytokeratins and vimentin and were hormone receptor and ERBB2 negative. CLDN membrane expression was negative in the spindle cell component. The epithelioid areas were CLDN-1 positive. Nuclear/cytoplasmatic expression of CLDN-4 was observed in all components, except in one case in which it was strongly expressed in the non-spindle areas. All three cases were CD44+/CD24−. E-cadherin was focally expressed in epithelioid cells, only in the squamous areas. Activating EGFR mutations were not found. One patient developed local recurrences, metastases and died. FLMCa have the immunohistochemical profile of claudin-low breast tumors, with low expression of adhesion molecules, presence of TIC and EMT phenotype. No EGFR activating mutations were found.









Similar content being viewed by others
References
Reis-Filho JS, Lakhani SR, Gobbi H, Sneige N (2012) Metaplastic carcinoma. WHO classification of tumours of the breast. International Agency for Research on Cancer, Lyon, pp 48–52
Gobbi H, Simpson JF, Borowsky A, Jensen RA, Page DL (1999) Metaplastic breast tumors with a dominant fibromatosis‐like phenotype have a high risk of local recurrence. Cancer 85(10):2170–2182
Sneige N, Yaziji H, Mandavilli SR, Perez ER, Ordonez NG, Gown AM, Ayala A (2001) Low-grade (fibromatosis-like) spindle cell carcinoma of the breast. Am J Surg Pathol 25(8):1009–1016
Reis‐Filho JS, Milanezi F, Steele D, Savage K, Simpson PT, Nesland J, Pereira EM, Lakhani SR, Schmitt FC (2006) Metaplastic breast carcinomas are basal‐like tumours. Histopathology 49(1):10–21
Kim M-J, Ro JY, Ahn S-H, Kim HH, Kim S-B, Gong G (2006) Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol 37(9):1217–1226
Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, Hernandez-Boussard T, Livasy C, Cowan D, Dressler L (2004) Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res 10(16):5367–5374
Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW (2010) Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci 107(35):15449–15454
Herschkowitz JI, Zhao W, Zhang M, Usary J, Murrow G, Edwards D, Knezevic J, Greene SB, Darr D, Troester MA (2012) Comparative oncogenomics identifies breast tumors enriched in functional tumor-initiating cells. Proc Natl Acad Sci 109(8):2778–2783
Prat A, Perou CM (2011) Deconstructing the molecular portraits of breast cancer. Mol Oncol 5(1):5–23
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM (2010) Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res 12(5):R68
Hennessy BT, Gonzalez-Angulo A-M, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee J-S, Fridlyand J, Sahin A, Agarwal R, Joy C (2009) Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res 69(10):4116–4124
Zhang Y, Toy KA, Kleer CG (2011) Metaplastic breast carcinomas are enriched in markers of tumor-initiating cells and epithelial to mesenchymal transition. Mod Pathol 25(2):178–184
Reis‐Filho JS, Pinheiro C, Lambros M, Milanezi F, Carvalho S, Savage K, Simpson PT, Jones C, Swift S, Mackay A (2006) EGFR amplification and lack of activating mutations in metaplastic breast carcinomas. J Pathol 209(4):445–453
Bhargava R, Gerald WL, Li AR, Pan Q, Lal P, Ladanyi M, Chen B (2005) EGFR gene amplification in breast cancer: correlation with epidermal growth factor receptor mRNA and protein expression and HER-2 status and absence of EGFR-activating mutations. Mod Pathol 18(8):1027–1033
Leibl S, Moinfar F (2005) Metaplastic breast carcinomas are negative for Her-2 but frequently express EGFR (Her-1): potential relevance to adjuvant treatment with EGFR tyrosine kinase inhibitors? J Clin Pathol 58(7):700–704
Reis-Filho JS, Milanezi F, Carvalho S, Simpson PT, Steele D, Savage K, Lambros M, Pereira EM, Nesland JM, Lakhani SR (2005) Metaplastic breast carcinomas exhibit EGFR, but not HER2, gene amplification and overexpression: immunohistochemical and chromogenic in situ hybridization analysis. Breast Cancer Res 7(6):R1028–R1035
Gerhard R, Ricardo S, Albergaria A, Gomes M, Silva AR, Logullo ÂF, Cameselle-Teijeiro JF, Paredes J, Schmitt F (2012) Immunohistochemical features of claudin-low intrinsic subtype in metaplastic breast carcinomas. Breast 21(3):354–360
Dunne B, Lee AH, Pinder SE, Bell JA, Ellis IO (2003) An immunohistochemical study of metaplastic spindle cell carcinoma, phyllodes tumor and fibromatosis of the breast. Hum Pathol 34(10):1009–1015
Lee A (2008) Recent developments in the histological diagnosis of spindle cell carcinoma, fibromatosis and phyllodes tumour of the breast. Histopathology 52(1):45–57
Nonnis R, Paliogiannis P, Giangrande D, Marras V, Trignano M (2012) Low-grade fibromatosis-like spindle cell metaplastic carcinoma of the breast: a case report and literature review. Clin Breast Cancer 12(2):147–150
Podetta M, D’Ambrosio G, Ferrari A, Sgarella A, Dal Bello B, Silvio Fossati G, Zonta S, Silini E, Dionigi P (2009) Low-grade fibromatosis-like spindle cell metaplastic carcinoma: a basal-like tumor with a favorable clinical outcome. Report of two cases. Tumori 95(2):264–267
Kinkor Z, Svitakova I, Ryska A, Kodet R, Hrabal P (2002) Metaplastic spindle-cell (fibromatosis-like) carcinoma of the breast—report of 4 cases. Cesk Patol 38(4):164–168
Pleşan D, Georgescu CV, Pătrană N, Pleşan C, Stoica D (2010) Immunohistochemical study of p53 and Ki67 in a group of patients with mammary carcinoma. Rom J Morphol Embryol Rev Roum Morphol Embryol 51(3):459
Gasco M, Shami S, Crook T (2002) The p53 pathway in breast cancer. Breast Cancer Res 4(2):70
Bartley AN, Ross DW (2002) Validation of p53 immunohistochemistry as a prognostic factor in breast cancer in clinical practice. Arch Pathol Lab Med 126(4):456–458
Conflict of interest
The authors declare no conflicts of interest in the work. No financial support was provided for the research presented in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rito, M., Schmitt, F., Pinto, A.E. et al. Fibromatosis-like metaplastic carcinoma of the breast has a claudin-low immunohistochemical phenotype. Virchows Arch 465, 185–191 (2014). https://doi.org/10.1007/s00428-014-1603-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1603-9