Abstract
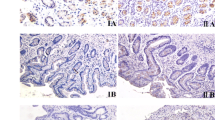
Although Helicobacter pylori is a risk factor for gastric cancer (GC), its detailed carcinogenesis remains unclear. Recently, aberrant expression of activation-induced cytidine deaminase (AID) was demonstrated in gastric epithelium with H. pylori infection and seems to cause the accumulation of mutation. This investigation aims to elucidate whether or not AID expression plays an important role in the carcinogenesis of early GC. We examined the correlation between immunohistochemical AID expression and histological characteristics, including pre-existing chronic gastritis and cellular mucin phenotype in 138 cases of intramucosal GC. Furthermore, we investigated the relationship between AID, p53 protein, and β-catenin. The low degree of polymorphonuclear neutrophil activity, and the high degree of glandular atrophy and intestinal metaplasia were significantly correlated with the high levels of AID expression in non-neoplastic mucosa (P = 0.007, P ≤ 0.001, and P = 0.003). With regard to mucin phenotype of carcinoma, the intestinal phenotype tended to have the higher AID expression levels (P = 0.052). AID showed close correlations with Cdx2 and nuclear staining of β-catenin (P = 0.003, P = 0.034). As for p53 protein, no correlation was found with AID expression. Our findings suggest that aberrant AID expression is correlated with persistent inflammatory condition induced by H. pylori infection and may contribute to the development of GC through an inflammatory condition and intestinalization.


Similar content being viewed by others
References
Lauren P (1965) The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Immunol Scand 64:31–49
Japanese Research Society of Gastric Cancer (2010) Japanese classification of gastric carcinoma, 14th edn. Kanehara, Tokyo, p 8
Correa P (1992) Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on cancer epidemiology and prevention. Cancer Res 52:6735–6740
IARC monographs on the evaluation of carcinogenic risks to humans (1994) Schistosomes, liver flukes and Helicobacter pylori. WHO Lyon 61:1–241
Uemura N, Okamoto S, Yamamoto S et al (2001) Helicobacter pylori infection and the development of gastric cancer. New Engl J Med 345:784–789
Tajima Y, Yamazaki K, Makino R et al (2006) Gastric and intestinal phenotypic marker expression in early differentiated type tumors of the stomach: clinicopathologic significance and genetic background. Clin Cancer Res 12:6469–6479
Sugiyama T (2004) Development of gastric cancer associated with Helicobacter pylori infection. Cancer Chemother Pharmacol 54(1):S12–S20
Maeda K, Onoda N, Ogawa M et al (1998) Expression of p53 and vascular endothelial growth factor associated with tumor angiogenesis and prognosis in gastric cancer. Oncology 55:594–599
Fenoglio-Preiser CM, Wang J, Stemmermann GN et al (2003) TP53 and gastric carcinoma: a review. Hum Mutat 21:258–270
Sasaki I, Yao T, Nawata H et al (1999) Minute gastric carcinoma of differentiated type with special reference to the significance of intestinal metaplasia, proliferative zone, and p53 protein during tumor development. Cancer 85:1719–1729
Muramatsu M, Sankaranand VS, Anant S et al (1999) Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J Biol Chem 274:18470–18476
Okazaki I, Hiai H, Kakazu N et al (2003) Constitutive expression of AID leads to tumorigenesis. J Exp Med 2:1173–1181
Kinoshita K, Nonaka T (2006) The dark side of activation-induced cytidine deaminase: relationship with leukemia and beyond. Int J Hematol 83:201–207
Shivarov V, Shinkura R, Doi T et al (2009) Molecular mechanism for generation of antibody memory. Phil Trans R Soc B 364:569–575
Endo Y, Marusawa H, Kou T, Nakase H et al (2008) Activation-induced cytidine deaminase between inflammation and the development of colitis-associated colorectal cancers. Gastroenterology 135:889–898
Endo Y, Marusawa H, Kinoshita K, Morisawa T et al (2007) Expression of activation-induced cytidine deaminase in human hepatocytes via NF-κB signaling. Oncogene 26:5587–5595
Komori J, Marusawa H, Machimoto T et al (2008) Activation-induced cytidine deaminase links bile duct inflammation to human cholangiocarcinoma. Hepatology 47:888–896
Matsumoto Y, Marusawa H, Kinoshita K et al (2007) Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med 13:470–476
Morisawa T, Marusawa H, Ueda Y et al (2008) Organ-specific profiles of genetic changes in cancers caused by activation-induced cytidine deaminase expression. Int J Cancer 123:2735–2740
Kim C, Song J, Cho Y et al (2007) Activation-induced cytidine deaminase expression in gastric cancer. Tumor Biol 339:333
Nakajima T, Yamaguchi T (eds) (2006) Gannkenn Igan Database 1946–2004 (Cancer Institute Hospital Gastric Cancer Database, 1946–2004) (in Japanese). Kanehara, Tokyo, 37
Kabashima A, Yao T, Sugimachi K et al (2000) Gastric or intestinal phenotypic expression in the carcinomas and background mucosa of multiple early gastric carcinomas. Histopathology 37:513–522
Dixon MF, Genta RM, Yardley JH et al (1996) The participants in the international workshop on the histopathology of gastritis, Houston 1994. Classification and grading of gastritis: the updated Sydney system. Am J Surg Pathol 20:1161–1181
Ito S, Nagaoka H, Shinkura R et al (2004) Activation-induced cytidine deaminase shuttles between nucleus and cytoplasm like apolipoprotein B mRNA editing catalytic polypeptide 1. PNAS 101:1975–1980
Kumashiro Y, Yao T, Aishima S et al (2007) Hepatoid adenocarcinoma of the stomach: histogenesis and progression in association with intestinal phenotype. Hum Pathol 38:857–863
Son HL, Hyun JK, Dong-Hun S et al (2009) Expression of beta-catenin and its mechanism of delocalization in intestinal-type early gastric cancer based on mucin expression. Histol Histopathol 24:831–838
Yao T, Akira K, Toshio K et al (1999) The phenotypes of the gastric carcinoma—evaluation by a new immunohistochemical method. Stomach Intestine 34:477–485
Grabsch H, Takeno S, Noguchi T et al (2001) Different patterns of b-catenin expression in gastric carcinomas: relationship with clinicopathological parameters and prognostic outcome. Histopathology 39:141–149
Kato S, Matsukura N, Tsukada K et al (2007) Helicobacter pylori infection-negative gastric cancer in Japanese hospital patients: incidence and pathological characteristics. Cancer Sci 98:790–794
Siano Y, Rugge M, Correa P et al (1994) p53 Alteration in gastric precancerous lesions. Am J Pathol 144:511–517
Ochiai A, Yamaguchi Y, Hirohasu S (1996) p53 Mutation in the non-neoplastic mucosa of the human stomach showing intestinal metaplasia. Int J Cancer 69:28–33
Mesquita P, Jonckheere N, Almeida R et al (2003) Human MUC2 mucin gene is transcriptionally regulated by Cdx homeodomain proteins in gastrointestinal carcinoma cell lines. J Biol Chem 278:51549–51556
Philip R, Debruyne, Matthew W et al (2006) Bile acids induce ectopic expression of intestinal guanylyl cyclase C through nuclear factor-kB and Cdx2 in human esophageal cells. Gastroenterology 130:1191–1206
Bai YQ, Miyake S, Iwai T et al (2003) CDX2, a homeobox transcription factor, upregulates transcription of the p21/WAF1/CIP1 gene. Oncogene 22:5998–6005
Takahashi K, Hirano F, Matsumoto K et al (2009) Homeobox gene CDX2 inhibits human pancreatic cancer cell proliferation by down-regulating cyclin D1 transcriptional activity. Pancreas 38:49–57
Kim HS, Lee JS, Freund JN et al (2006) CDX-2 homeobox gene expression in human gastric carcinoma and precursor lesions. J Gastroenterol Hepatol 21:438–442
Mutoh H, Sakurai S, Satoh K et al (2004) Development of gastric carcinoma from intestinal metaplasia in Cdx2-transgenic mice. Cancer Res 64:7740–7747
Acknowledgments
The authors thank Ms. Y. Kamitani for her technical assistance. The English used in the manuscript was revised by Miss K. Miller (Royal English Language Centre, Fukuoka, Japan).
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goto, A., Hirahashi, M., Osada, M. et al. Aberrant activation-induced cytidine deaminase expression is associated with mucosal intestinalization in the early stage of gastric cancer. Virchows Arch 458, 717–724 (2011). https://doi.org/10.1007/s00428-011-1086-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-011-1086-x