Abstract
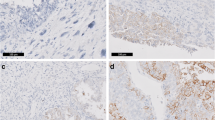
This study was designed to analyze the subcellular localization of E-cadherin and β-catenin both of which play a critical role in cell–cell adhesion in uterine carcinosarcoma (UCS). We performed an immunohistochemical reaction analysis of the subcellular localization of E-cadherin and β-catenin proteins in 46 cases of UCSs consisting of 28 UCSs with heterologous sarcoma and 18 UCSs with homologous sarcoma and compared their clinicopathological features. In most UCSs, membranous expression of E-cadherin and β-catenin was completely lost in sarcomatous components, but it was preserved in carcinomatous components. Nuclear β-catenin expression was observed significantly more frequently in sarcomatous components (31/46, 67.4%) than in carcinomatous components (22/46, 47.8%; P = 0.0025). In sarcomatous components, nuclear β-catenin expression was found significantly more frequently in heterologous sarcoma (23/28, 82.1%) than in homologous sarcoma (8/18, 44.4%; P = 0.0279). The stage was the only independent prognostic significant factor. These results suggest that reduced membranous expression of E-cadherin and β-catenin may contribute to the biphasic morphology of UCS. Furthermore, although the precise mechanism is unclear, nuclear β-catenin expression in sarcomatous components may also be associated with biphasic morphology and heterologous sarcomatous differentiation.









Similar content being viewed by others
References
Silverberg SG, Major FJ, Blessing JA et al (1990) Carcinosarcoma (malignant mixed mesodernal tumor) of the uterus. A gynecologic oncology group pathologic study of 203 cases. Int J Gynecol Pathol 9:1–19
Wada H, Enomoto T, Fujita M et al (1997) Molecular evidence that most but not all carcinosarcomas of the uterus are combination tumors. Cancer Res 57:5379–5385
Emoto M, Iwasaki H, Kikuchi M, Shirakawa K (1993) Characteristics of cloned cells of mixed mullerian tumor of the human uterus. Carcinoma cells showing myogenic differentiation in vitro. Cancer 71:3065–3075
Fujii H, Yoshida M, Gong ZX et al (2000) Frequent genetic heterogeneity in the clonal evolution of gynecological carcinosarcoma and its influence on phenotypic diversity. Cancer Res 60:114–120
McCluggage WG (2002) Uterine carcinosarcomas (malignant mixed mullerian tumors) are metaplastic carcinomas. Int J Gynecol Cancer 12:687–690
Sreenan JJ, Hart WR (1995) Carcinosarcomas of the female genital tract. A pathologic study of 29 metastatic tumors: further evidence for the dominant role of the epithelial component and the conversion theory of histogenesis. Am J Surg Pathol 19:666–674
Ferguson SE, Tornos C, Hummer A, Barakat RR, Soslow RA (2007) Prognostic features of surgical stage I uterine carcinosarcoma. Am J Surg Pathol 31:1653–1661
Goltzmann J, Mikula M, Eger A et al (2004) Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res 566:9–20
Sarrió D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J (2006) Epithelial–mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res 68:989–997
Thiery JP (2002) Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Mayer B, Johnson JP, Leitl F et al (1993) E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res 53:1690–1695
Saito T, Oda Y, Sugimachi K et al (2001) E-cadherin gene mutations frequently occur in synovial sarcoma as a determinant of histological features. Am J Pathol 159:2117–2124
Gumbiner BM (2000) Regulation of cadherin adhesive activity. J Cell Biol 148:399–404
Saito T, Oda Y, Sakamoto A et al (2000) Prognostic value of the preserved expression of the E-cadherin and catenin families of adhesion molecules and of β-catenin mutations in synovial sarcoma. J Pathol 192:342–350
Shimazui T, Schalken JA, Giroldi LA et al (1996) Prognostic value of cadherin-associated molecules (α-, β-, and γ-catenins and p120cas) in bladder tumors. Cancer Res 56:4154–4158
Ilyas M (2005) Wnt signalling and the mechanistic basis of tumour development. J Pathol 205:130–144
Tetsu O, McCormick F (1999) β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426
Chen G, Shukeir N, Potti A et al (2004) Up-regulation of Wnt-1 and β-catenin production in patients with advanced metastatic prostate carcinoma. Potential pathologenetic and prognostic implications. Cancer 101:1345–1356
Fukuchi T, Sakamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S (1998) β-catenin mutation in carcinoma of the uterine endometrium. Cancer Res 58:3526–3528
Saegusa M, Hashimura T, Yoshida T, Okayasu I (2001) β-catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer 84:209–217
Scholten AN, Creutzberg CL, van den Broek LJCM, Noordijk EM, Smit VTHBM (2003) Nuclear β-catenin is a molecular feature of type I endometrial carcinoma. J Pathol 201:460–465
Kondo Y, Kanai Y, Sakamoto M et al (1999) β-catenin accumulation and mutation of exon 3 of the β-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res 90:1301–1309
Van Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA (1999) Nuclear accumulation of mutated β-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol 155:703–710
Eger A, Stockinger A, Park J et al (2004) β-catenin and TGFβ signalling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene 23:2672–2680
Moreno-Bueno G, Hardisson D, Sarrio D et al (2003) Abnormalities of E- and P-cadherin and catenin (β-, γ-catenin, and p120ctn) expression in endometrial cancer and endometrial atypical hyperplasia. J Pathol 199:471–478
Scholten AN, Aliredjo R, Creutzberg CL, Smit VTHBM (2006) Combined E-cadherin, α-catenin, and β-catenin expression is a favorable prognostic factor in endometrial carcinoma. Int J Gynecol Cancer 16:1379–1385
Nikaido T, Li SF, Shiozawa T, Fujii S (1996) Coabnormal expression of cyclin D1 and p53 protein in human uterine endometrial carcinomas. Cancer 78:1248–1253
Saegusa M, Hashimura M, Kuwata T, Okayasu I (2009) Requirement of the Akt/β-catenin pathway for uterine carcinosarcoma genesis, modulating E-cadherin expression through the transactivation of Slug. Am J Pathol 174:2107–2115
Kirchner T, Brabletz T (2000) Patterning and nuclear beta-catenin expression in the colonic adenoma-carcinoma sequence. Analogies with embryonic gastrulation. Am J Pathol 157:1113–1121
Fodde R, Brabletz T (2007) Wnt/β-catenin signaling in cancer stemness and malignant behavior. Cur Op Cell Biol 19:150–158
Van Es JH, Barker N, Clevers H (2003) You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev 13:28–33
Petropoulos H, Skerjanc IS (2002) β-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J Biol Chem 277:15393–15399
Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 10:55–63
Matsushime H, Ewen ME, Strom DK et al (1992) Identification and properties of an atypical catalytic subunit (p34psk-j3/CDK4) for mammalian D type G1 cyclins. Cell 71:323–334
Ozaki S, Ikeda S, Ishizaki Y et al (2005) Alterations and correlations of the components in the Wnt signaling pathway and its target genes in breast cancer. Oncol Rep 14:1437–1443
Iwasa Y, Haga H, Konishi I et al (1998) Prognostic factors in uterine carcinosarcoma. A clinicopathologic study of 25 patients. Cancer 82:512–519
Acknowledgments
We are grateful to Yoko Kamitani for her excellent technical assistance. We thank KN International for revising the English used in this article.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishimura, I., Ohishi, Y., Oda, Y. et al. Expression and localization of E-cadherin and β-catenin in uterine carcinosarcoma. Virchows Arch 458, 85–94 (2011). https://doi.org/10.1007/s00428-010-1002-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-1002-9