Abstract
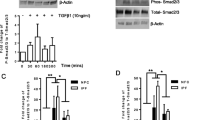
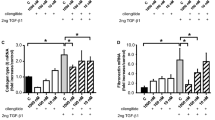
Pulmonary fibrosis is a common feature of a large group of lung diseases. The molecular mechanisms underlying pulmonary fibrosis and the key macromolecules involved are not fully understood yet. In an effort to better understand aspects of pulmonary fibrosis, the established bleomycin injection model in mice was used and the focus of the present study was on integrin-linked kinase (ILK) expression. ILK is an intracellular protein involved in the regulation of integrin-mediated processes. In fibrosis, ILK has been examined in the kidney and in the liver where it mediates epithelial to mesenchymal transition (EMT) and hepatic stellate cell activation, respectively. However, information on ILK’s involvement in lung fibrosis is missing. In order to examine ILK’s role in pulmonary fibrosis, we used both an in vivo and an in vitro approach. In vivo, the bleomycin model was used in order to examine ILK’s expression and localization in the fibrotic lung. In vitro, transforming growth factor-β1 was used to induce fibrotic characteristics and EMT in alveolar epithelial cells. ILK’s role in alveolar EMT was studied by siRNA. Our results demonstrate that in the animal model used, ILK exhibits a decrease in expression at early stages of the fibrotic process and that a specific subset of fibroblasts is expressing ILK. The in vitro experiments suggested that ILK is not directly involved in E-cadherin downregulation and initiation of EMT (as is the case in renal fibrosis) but is involved in upregulation of vimentin. These results suggest that ILK is involved in lung fibrosis in a tissue-specific manner and raise the possibility to use it as a specific therapeutic target for lung fibrosis in the future.









Similar content being viewed by others
References
Green F (2002) Overview of pulmonary fibrosis. Chest 122:334–339
Coultas DB, Zumwalt RE, Black WE, Sobonya RE (1994) The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med 150:967–972
American Thoracic Society ERS (2000) Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. Am J Respir Crit Care Med 161:646–664
Moore BB, Hogaboam CM (2008) Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 294:L152–L160
Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ (1997) Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol 29:5–17
Kisseleva T, Brenner DA (2008) Mechanisms of fibrogenesis. Exp Biol Med 233:109–122
Kumar V, Abbas AK, Fausto N (2005) Tissue renewal and repair: regeneration, healing and fibrosis. In: Kumar V, Abbas AK, Fausto N (eds) Pathologic basis of disease, 7th edn. Elsevier, Saunders, pp 87–118
Kalluri R, Neilson EG (2003) Epithelial–mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1:71–81
Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, Sherrill TP, Plieth D, Neilson EG, Blackwell TS, Lawson WE (2009) Contribution of epithelial derived fibroblasts to bleomycin induced lung fibrosis. Am J Respir Crit Care Med 180(7):657–665
Wu Z, Yang L, Cai L, Zhang M, Cheng X, Yang X, Xu J (2007) Detection of epithelial to mesenchymal transition in airways of a bleomycin induced pulmonary fibrosis model derived from an α-smooth muscle actin-Cre transgenic mouse. Respir Res 8:1–11
Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA (2006) Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. PNAS 103(35):13180–13185
Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J (1996) Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379:91–96
Legate KR, Montanez E, Kudlacek O, Fassler R (2006) ILK, PINCH and parvin: the Tipp of integrin signaling. Nature 7:20–31
Wu C, Keightly SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S (1998) Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression and tumorigenenicity. J Biol Chem 273:528–536
Persad S, Attwell S, Gray V, Mawji N, Deng JT, Leung D (2001) Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem 276:27462–27469
Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/Akt by the integrin-linked kinase. Proc Natl Acad Sci 95:11211–11216
Li Y, Yang J, Dai C, Wu C, Liu Y (2003) Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Investig 112:503–516
Li Y, Dai C, Wu C, Liu Y (2007) PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J Am Soc Nephrol 18:2534–2543
Gkretsi V, Mars WM, Bowen WC, Barua L, Yang Y, Guo L, St-Arnaud R, Dedhar S, Wu C, Michalopoulos GK (2007) Loss of integrin-linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology 45(4):1025–1034
Zhang Y, Ikegami T, Honda A, Miyazaki T, Bouscarel B, Rojkind M, Hyodo I, Matsuzaki Y (2006) Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology 44(3):612–622
Chaudhary NI, Schnapp A, Park JE (2005) Pharmacological differentiation of inflammation and fibrosis in the rat bleomycin model. Am J Respir Crit Care Med 173:769–776
Izbicki G, Segel MJ, Christensen TG, Conner MW, Breuer R (2002) Time course of bleomycin-induced lung fibrosis. Int J Exp Path 83:111–119
Adamson IY, Bowden DH (1974) The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest 30:35–42
Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS (2005) Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med 171:889–907
Li Y, Tan X, Dai C, Stolz DB, Wang D, Liu Y (2009) Inhibition of integrin-linked kinase attenuates renal interstitial fibrosis. J Am Soc Nephrol 20(9):1907–1918
Bowden DH (1981) Alveolar response to injury. Thorax 36:801–804
Hegab AE, Kubo H, Fujino N, Suzuki T, He M, Kato H, Yamaha M (2010) Isolation and characterization of murine multipotent lung stem cells. Stem Cells Dev 19(4):523–526
Flier SN, Tanjore H, Kokkotou EG, Sugimoto H, Zeisberg M, Kalluri R (2010) Identification of epithelial to mesenchymal transition as a novel source of fibroblasts in intestinal fibrosis. J Biol Chem 285(26):20202–20212
Moeller A, Ask K, Warburton D, Gauldie J, Kolb M (2008) The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol 40(3):362–382
Hardie WD, Glasser SW, Hagood JS (2009) Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol 175(1):3–16
Gilles G, Polette M, Piette J, Delvigne AC, Thompson EW, Foidart JM, Birembaut P (1996) Vimentin expression in cervical carcinomas: association with invasive and migratory potential. J Pathol 180(2):175–180
Paccione RJ, Miyazaki H, Patel V, Waseem A, Gutkind JS, Zehner ZE, Yeudall WA (2008) Keratin down-regulation in vimentin-positive cancer cells is reversible by vimentin RNA interference, which inhibits growth and motility. Mol Cancer Ther 7(9):2894–2903
Acknowledgments
The present work was supported by funds from Biomedical Research Foundation of the Academy of Athens and PENED 03ED245 from the Greek Secretariat for Research and Technology. We are grateful to Dr. Stavros Giaglis for help with RT-RT-PCR, Dr. Stamatis N. Pagakis and Dr. Eleni Rigana for their help with confocal microscopy, Anna Agapaki for help with histological techniques, and to the members of Department of Histology for the excellent cooperation. We are also thankful to Dr. Dimitrios Kouretas for his support and interaction.
Declaration of interest
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kavvadas, P., Kypreou, K.P., Protopapadakis, E. et al. Integrin-linked kinase (ILK) in pulmonary fibrosis. Virchows Arch 457, 563–575 (2010). https://doi.org/10.1007/s00428-010-0976-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-010-0976-7