Abstract
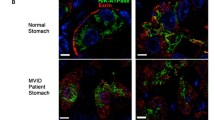
Microvillous Inclusion Disease (MID) is an inherited disorder characterized by intractable diarrhea in infancy. Ultrastructural detection of pathognomonic microvillous inclusions in the enterocytes is essential for diagnosis. The aim of this research is to contribute to the knowledge of MID studying enterocytes and goblet cells (gc). Samples of duodenal mucosa from two young infants with MID (aged 75 days and 3 months, respectively) were studied by light and electron microscopy. Detection in the intestinal villi of immature gc (with microvilli) in one of the cases led us to seek them in control samples. The total number of gc with microvilli (immature) and without microvilli (mature) were counted. In both MID specimens, light microscopy showed atrophy of villi and PAS-positive material in the enterocyte cytoplasm. The ultrastructure of villous enterocytes was characterized by brush-border abnormalities, microvillous inclusions, dense apical granules, and lysosomes. Intermediate structures between microvillous inclusions and lysosomes were also detected within a cell, as were rare microvilli on the lateral membrane of the enterocytes. In one MID specimen, immature gc were also identified in the absorptive compartment. Only mature gc were observed in the controls. The significance of the latter finding requires further studies.






Similar content being viewed by others
References
Afzelius BA (1984) Glycocalix and glycocalyceal bodies in the respiratory epithelium of nose and bronchi. Ultrastruct Pathol 7:1–8
Ameen NA, Salas PJI (2000) Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic 1:76–83
Bell SW, Kerner JA, Sibley RK (1991) Microvillous inclusion disease. The importance of electron microscopy for diagnosis. Am J Surg Pathol 15:1157–1164
Carruthers L, Dourmanshkin R, Phillips AD (1985) Disorders of the cytoskeleton of the enterocyte. Clin Gastroenterol 15:105–120
Carruthers L, Phillips AD, Dourmanshkin R, Walker-Smith JA (1986) Biochemical abnormality in brush border membrane protein of a patient with congenital microvillous atrophy. J Pediatr Gastroenterol Nutr 4:902–907
Croft NM, Howatson AG, Ling SC, Nairn L, Evans TJ, Weaver LT (2000) Microvillous inclusion disease: an evolving condition. J Pediatr Gastroenterol Nutr 31:185–189
Cutz E, Rhoads JM, Drumm B, Sherman PM, Durie PR, Forstner GG (1989) Microvillus inclusion disease: an inherited defect of brush-border assembly and differentiation. N Engl J Med 320:646–651
Davidson GP, Cutz E, Hamilton JR, Gall DG (1978) Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive and hypoplastic villus atrophy. Gastroenterology 75:783–790
Ellinger A, Gruber K, Stockinger L (1987) Glycocalyceal bodies—a marker for different epithelial cell types in human airways. J Submicrosc Cytol 19:311–320
Erlandson RA (ed) (1994) Intestinal-type microvilli. In: Diagnostic transmission electron microscopy of tumors. Raven, New York, pp 202–204
Fawcett DW (ed) (1994) Intestines. In: Bloom and Fawcett, A textbook of histology, 12th edn. Chapman & Hall, New York, pp 617–651
Freeman JA (1966) Goblet cell fine structure. Anat Rec 154:121–130
Ghadially FN (ed) (1985) Filamentous core rootlets and glycocalyceal bodies. In: Diagnostic electron microscopy of tumors, 2nd edn. Butterworths, London, pp 334–342
Ghadially FN (ed) (1997) Glycocalyceal bodies and filamentous core rootlets. In: Ultrastructural pathology of the cell and matrix, 4th edn. Butterworth-Heinemann, Boston, pp 1144–1151
Guandalini S, Nocerino A (2002) Congenital microvillus atrophy. Available at: URL http://www.emedicine.com/ped/topic461.htm. Last update May 25
Kučinskienė R, Jančiauskas D, Pužzs A, Adamonis K (2004) Microvillous inclusion disease. Medicina (Kaunas) 40:864–867
Marcus PB, Martin JH, Green RH, Krouse MA (1979) Glycocalyceal bodies and microvillous core rootlets. Arch Pathol Lab Med 103:89–92
Michail S, Collins JF, Xu H, Kaufman S, Vanderhoof J, Ghishan FK (1998) Abnormal expression of brush-border membrane transporters in the duodenal mucosa of two patients with microvillus inclusion disease. J Pediatr Gastroenterol Nutr 27:536–542
Mierau GW, Willis EJ, Wyatt-Ashmead J, Hoffenberg EJ, Cutz E (2001) Microvillous inclusion disease: report of a case with atypical features. Ultrastruct Pathol 25:517–521
Ozzello L, Savary M, Roethlisberger B (1977) Columnar mucosa of the distal esophagus in patients with gastroesophageal reflux. Pathol Annu 12:41–86
Pavelka MP, Gangl A (1983) Effects of colchicines on the intestinal transport of endogenous lipid. Gastroenterology 84:544–555
Phillips AD, Jenkins P, Raafat F, Walker-Smith JA (1985) Congenital microvillous atrophy: specific diagnostic features. Arch Dis Child 60:135–140
Phillips AD, Schmitz J (1992) Familial microvillous atrophy: a clinicopathological survey of 23 cases. J Pediatr Gastroenterol Nutr 14:380–396
Phillips AD, Szafranski M, Man L-Y, Wall WJ (2000) Periodic acid-Schiff staining abnormality in microvillous atrophy: photometric and ultrastructural studies. J Pediatr Gastroenterol Nutr 30:34–42
Phillips AD, Brown A, Swallow DM, Hicks S, Schüller S, Murch SH, Walker-Smith JA (2004) Acetylated sialic acid residues and blood group antigens localise within the epithelium in microvillous atrophy indicating internal accumulation of the glycocalyx. Gut 53:1764–1771
Pohl JF, Shub MD, Trevelline EE, Ingebo K, Silber G, Rayhorn N, Holve S, Hu D (1999) A cluster of microvillous inclusion disease in the Navajo population. J Pediatr 134:103–106
Raafat F, Green NJ, Nathavitharana KA, Booth IW (1994) Intestinal microvillous dystrophy: a variant of microvillous inclusion disease or a new entity? Hum Pathol 25:1243–1248
Reinshagen K, Naim HY, Zimmer KP (2002) Autophagocytosis of the apical membrane in microvillus inclusion disease. Gut 51:514–521
Ruemmele FM, Jan D, Lacaille F, Cézard J-P, Canioni D, Phillips AD, Peuchmaur M, Aigrain Y, Brousse N, Schmitz J, Revillon Y, Goulet O (2004) New perspectives for children with microvillous inclusion disease: early small bowel transplantation. Transplantation 77:1024–1028
Sandoz D, Nicolas G, Laine M-C (1985) Two mucous cell types revisited after quick-freezing. Biol Cell 54:79–99
Schofield DE, Agostini RM, Yunis EJ (1992) Gastrointestinal microvillus inclusion disease. Am J Clin Pathol 98:119–124
Shaw RJ, Henry M, Solomon F, Jacks T (1998) RhoA-dependent phosphorylation and relocalization of ERM proteins into apical membrane/actin protrusion in fibroblasts. Mol Biol Cell 9:403–419
Weeks DA, Zuppan CW, Malott RL, Mierau GW (2003) Microvillous inclusion disease with abundant vermiform, electron-lucent vesicles. Ultrastruct Pathol 27:337–340
Acknowledgements
We are grateful to Drs. Mariella Marelli and Maria Cristina Zingaretti for their excellent technical assistance and to Dr. Michele Bisceglia for his critical reading and comments on the manuscript. This study was supported by a grant from the Polytechnic University of Marche (2005 FAR, formerly 60%) to M. M.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morroni, M., Cangiotti, A., Guarino, A. et al. Unusual ultrastructural features in microvillous inclusion disease: a report of two cases. Virchows Arch 448, 805–810 (2006). https://doi.org/10.1007/s00428-006-0180-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-006-0180-y