Abstract
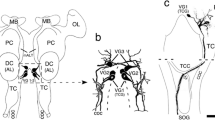
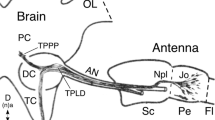
The central complex comprises an elaborate system of modular neuropils which mediate spatial orientation and sensory-motor integration in insects such as the grasshopper and Drosophila. The neuroarchitecture of the largest of these modules, the fan-shaped body, is characterized by its stereotypic set of decussating fiber bundles. These are generated during development by axons from four homologous protocerebral lineages which enter the commissural system and subsequently decussate at stereotypic locations across the brain midline. Since the commissural organization prior to fan-shaped body formation has not been previously analyzed in either species, it was not clear how the decussating bundles relate to individual lineages, or if the projection pattern is conserved across species. In this study, we trace the axonal projections from the homologous central complex lineages into the commissural system of the embryonic and larval brains of both the grasshopper and Drosophila. Projections into the primordial commissures of both species are found to be lineage-specific and allow putatively equivalent fascicles to be identified. Comparison of the projection pattern before and after the commencement of axon decussation in both species reveals that equivalent commissural fascicles are involved in generating the columnar neuroarchitecture of the fan-shaped body. Further, the tract-specific columns in both the grasshopper and Drosophila can be shown to contain axons from identical combinations of central complex lineages, suggesting that this columnar neuroarchitecture is also conserved.







Similar content being viewed by others
References
Ashburner M (1989) Drosophila: a laboratory handbook and manual. Cold Spring Harbor Laboratory, New York
Bello BC, Izergina N, Caussinus E, Reichert H (2008) Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev 3:5
Bentley D, Keshishian H, Shankland M, Torian-Raymond A (1979) Quantitative staging of embryonic development of the grasshopper, Schistocerca nitens. J Embryol Exp Morphol 54:47–74
Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS (1989) Drosophila neuroglian: a member of the immunoglobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell 59:447–460
Böhm A, Szucsich NU, Pass G (2012) Brain anatomy in Diplura (Hexapoda). Front Zool 9:26
Boyan GS, Reichert H (2011) Mechanisms for complexity in the brain: generating the insect central complex. Trends Neurosci 34:247–257
Boyan GS, Williams JLD (1997) Embryonic development of the pars intercerebralis/central complex of the grasshopper. Dev Genes Evol 207:317–329
Boyan GS, Williams L (2011) Embryonic development of the insect central complex: insights from lineages in the grasshopper and Drosophila. Arthropod Struct Dev 40:334–348
Boyan GS, Liu Y (2016) Development of the neurochemical architecture of the central complex. Front Behav Neurosci 10:1
Boyan GS, Williams JLD, Herbert Z (2008a) An ontogenetic analysis of locustatachykinin-like expression in the central complex of the grasshopper Schistocerca gregaria. Arthropod Struct Dev 37:480–491
Boyan GS, Williams JL, Herbert Z (2008b) Fascicle switching generates a chiasmal neuroarchitecture in the embryonic central body of the grasshopper Schistocerca gregaria. Arthropod Struct Dev 37:539–544
Boyan G, Williams L, Liu Y (2015) Conserved patterns of axogenesis in the panarthropod brain. Arthropod Struct Dev 44:101–112
Boyan G, Williams L, Meier T (1993) Organization of the commissural fibers in the adult brain of the locust. J Comp Neurol 332:358–377
Boyan GS, Therianos S, Williams JLD, Reichert H (1995) Axogenesis in the embryonic brain of the grasshopper Schistocerca gregaria: an identified cell analysis of early brain development. Development 121:75–86
Campos-Ortega JA, Hartenstein VH (1997) The embryonic development of Drosophila melanogaster, 2nd edn. Springer, Berlin, Heidelberg, New York
Cardona A, Saalfeld S, Arganda I, Pereanu W, Schindelin J, Hartenstein V (2010) Identifying neuronal lineages of Drosophila by sequence analysis of axon tracts. J Neurosci 30:7538–7553
de Velasco B, Erclik T, Shy D, Sclafani J, Lipschitz H, McInnes R, Hartenstein V (2007) Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev Biol 302:309–323
el Jundi B, Heinze S, Lenschow C, Kurylas A, Rohlfing T, Homberg U (2010) The locust standard brain: a 3D standard of the central complex as a platform for neural network analysis. Front Syst Neurosci 3:21
Goodman CS, Doe CQ (1994) Embryonic development of the Drosophila central nervous system. In: Bate M, Martinez-Arias A (eds) The development of Drosophila, vol 1. Cold Spring Harbor Press, New York, pp 1131–1206
Harley CM, Ritzmann RE (2010) Electrolytic lesions within central complex neuropils of the cockroach brain affect negotiation of barriers. J Exp Biol 231:2851–2864
Hartenstein V, Younossi-Hartenstein A, Lovick JK, Kong A, Omoto JJ, Ngo KT, Viktorin G (2015) Lineage-associated tracts defining the anatomy of the Drosophila first instar larval brain. Dev Biol 406:14–39
Heinze S, Homberg U (2007) Maplike representation of celestial e-vector orientations in the brain of an insect. Science 315:995–997
Heinze S, Homberg U (2008) Neuroarchitecture of the central complex of the desert locust: intrinsic and columnar neurons. J Comp Neurol 511:454–478
Hidalgo A, Booth GE (2000) Glia dictate pioneer axon trajectories in the Drosophila embryonic CNS. Development 127:393–402
Homberg U (1987) Structure and functions of the central complex in insects. In: Gupta AP (ed) Arthropod brain: its evolution, development, structure, and functions. Wiley Press, New York, pp 347–367
Hortsch M, Patel NH, Bieber AJ, Traquina ZR, Goodman CS (1990) Drosophila neurotactin, a surface glycoprotein with homology to serine esterases, is dynamically expressed during embryogenesis. Development 110:1327–1340
Hummel T, Schimmelpfeng K, Klämbt C (1999) Commissure formation in the embryonic CNS of Drosophila. II Function of the different midline cells. Development 126:771–779
Ilius M, Wolf R, Heisenberg M (1994) The central complex of Drosophila melanogaster is involved in flight control: studies on mutants and mosaics of the gene ellipsoid body open. J Neurogenet 9:189–206
Ito K, Hotta Y (1992) Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol 149:134–148
Ito K, Awasaki T (2008) Clonal unit architecture of the adult fly brain. In: Technau GM (ed) Brain development in Drosophila melanogaster. Springer, New York, pp 137–158
Ito M, Masuda N, Shinomiya K, Endo K, Ito K (2013) Systematic analysis of neural projections reveals clonal composition of the Drosophila brain. Curr Biol 23:644–655
Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, Keshishian H, Restifo LL, Rössler W, Simpson JH, Strausfeld NJ, Strauss R, Vosshall LB (2014) A systematic nomenclature for the insect brain. Neuron 81:755–765
Iwai Y, Usui T, Hirano S, Steward R, Takeichi M, Uemura T (1997) Axon patterning requires DN-cadherin, a novel neuronal adhesion receptor, in the Drosophila embryonic CNS. Neuron 19:77–89
Jacobs JR, Goodman CS (1989) Embryonic development of axon pathways in the Drosophila CNS. I A glial scaffold appears before the first growth cones. J Neurosci 9:2402–2411
Kaprielian Z, Runko E, Imondi R (2001) Axon guidance at the midline choice point. Dev Dyn 221:154–181
Koniszewski NDB, Kollmann M, Bigham M, Farnworth M, He B, Büscher M, Hütteroth W, Binzer M, Schachtner J, Bucher G (2016) The insect central complex as model for heterochronic brain development—background, concepts, and tools. Dev Genes Evol 226:209–219
Liu G, Seiler H, Wen A, Zars T, Ito K, Wolf M, Heisenberg M, Liu L (2006) Distinct memory traces for two visual features in the Drosophila brain. Nature 439:551–556
Loesel R, Nässel DR, Strausfeld NJ (2002) Common design in a unique midline neuropil in the brains of arthropods. Arthropod Struct Dev 31:77–91
Lovick JK, Ngo KT, Omoto JJ, Wong DC, Nguyen JD, Hartenstein V (2013) Postembryonic lineages of the Drosophila brain: I. Development of the lineage-associated fiber tracts. Dev Biol 384:228–257
Lovick JK, Hartenstein V (2015) Hydroxyurea-mediated neuroblast ablation establishes birth dates of secondary lineages and addresses neuronal interactions in the developing Drosophila brain. Dev Biol 402:32–47
Lovick JK, Omoto JJ, Ngo KT, Hartenstein V (2017) Development of the anterior visual input pathway to the Drosophila central complex. J Comp Neurol (in press)
Naimski P, Bierzyimageski A, Fikus M (1980) Quantitative fluorescent analysis of different conformational forms of DNA bound to the dye 4′, 6-diamidine-2-phenylindole, and separated by gel electrophoresis. Anal Biochem 106:471–475
Nassif C, Noveen A, Hartenstein V (1998) Embryonic development of the Drosophila brain I. The pattern of pioneer tracts. J Comp Neurol 402:10–31
Nassif C, Noveen A, Hartenstein V (2003) Early development of the Drosophila brain III. The pattern of neuropile founder tracts during the larval period. J Comp Neurol 455:417–434
Neuser K, Triphan T, Mronz M, Poeck B, Strauss R (2008) Analysis of a spatial orientation memory in Drosophila. Nature 453:1244–1247
Noordermeer JN, Kopczynski CC, Fetter RD, Bland KS, Chen WY, Goodman CS (1998) Wrapper, a novel member of the Ig superfamily, is expressed by midline glia and is required for them to enshealth commissural axons in Drosophila. Neuron 21:991–1001
Page DT (2004) A mode of arthropod brain evolution suggested by Drosophila commissure development. Evol Dev 6:25–31
Pan Y, Zhou Y, Guo C, Gong H, Gong Z, Liu L (2009) Differential roles of the fan-shaped body and the ellipsoid body in Drosophila visual pattern memory. Learn Mem 16:289–295
Pereanu W, Hartenstein V (2006) Neural lineages of the Drosophila brain: a three-dimensional digital atlas of the pattern of lineage location and projection at the late larval stage. J Neurosci 26:5534–5553
Pereanu W, Kumar A, Jennett A, Reichert H, Hartenstein V (2010) Development-based compartmentalization of the Drosophila central brain. J Comp Neurol 518:2996–3023
Pfeiffer K, Homberg U (2014) Organization and functional roles of the central complex in the insect brain. Annu Rev Entomol 59:165–184
Piovant M, Léna P (1988) Membrane glycoproteins immunologically related to the human insulin receptor are associated with presumptive neuronal territories and developing neurones in Drosophila melanogaster. Development 103:145–156
Reichert H, Boyan GS (1997) Building a brain: insights from insects. Trends Neurosci 20:258–264
Renn SCN, Armstrong JD, Yang M, Wang Z, An X, Kaiser K, Taghert PH (1999) Genetic analysis of the Drosophila ellipsoid body neuropil: organization and development of the central complex. J Neurobiol 41:189–207
Riebli N, Viktorin G, Reichert H (2013) Early-born neurons in type II neuroblast lineages establish a larval primordium and integrate into adult circuitry during central complex development in Drosophila. Neural Dev 8:6
Schwabe T, Borycz JA, Meinertzhagen IA, Clandinin TR (2014) Differential adhesion determines the organization of synaptic fascicles in the Drosophila visual system. Curr Biol 24:1304–1313
Seeger M, Tear G, Ferres-Marco D, Goodman CS (1993) Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron 10:409–426
Snow PM, Bieber AJ, Goodman CS (1989) Fasciclin III: a novel homophilic adhesion molecule in Drosophila. Cell 59:313–323
Strausfeld NJ (2012) Arthropod brains. Harvard Univ Press, Cambridge
Strausfeld NJ, Hirth F (2013) Deep homology of arthropod central complex and vertebrate basal ganglia. Science 340:157–161
Strauss R (2002) The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol 12:633–638
Therianos S, Leuzinger S, Hirth F, Goodman CS, Reichert H (1995) Embryonic development of the Drosophila brain: formation of commissural and descending pathways. Development 121:3849–3860
Vitzthum H, Homberg U (1998) Immunocytochemical demonstration of locustatachykinin-related peptides in the central complex of the locust brain. J Comp Neurol 390:455–469
Wegerhoff R, Breidbach O (1992) Structure and development of the larval central complex in a holometabolous insect, the beetle Tenebrio molitor. Cell Tissue Res 268:341–358
Wegerhoff R, Breidbach O, Lobemeier M (1996) Development of locustatachykinin immunopositive neurons in the central complex of the beetle Tenebrio molitor. J Comp Neurol 375:157–166
Williams JLD (1975) Anatomical studies of the insect central nervous system: a ground-plan of the midbrain and an introduction to the central complex in the locust, Schistocerca gregaria (Orthoptera). J Zool Lond 176:67–86
Williams JLD, Boyan GS (2008) Building the central complex of the grasshopper Schistocerca gregaria: axons pioneering the w, x, y, z tracts project onto the primary commissural fascicle of the brain. Arthropod Struct Dev 37:129–140
Williams JLD, Guentner M, Boyan GS (2005) Building the central complex of the grasshopper Schistocerca gregaria: temporal topology organizes the neuroarchitecture of the w, x, y, z tracts. Arthropod Struct Dev 34:97–110
Wolff T, Iyer NA, Rubin GM (2015) Neuroarchitecture and neuroanatomy of the Drosophila central complex: a GAL4-based dissection of protocerebral bridge neurons and circuits. J Comp Neurol 523:997–1037
Wong DC, Lovick JK, Ngo KT, Omoto JJ, Borisuthirattana W, Hartenstein V (2013) Postembryonic lineages of the Drosophila brain: II. Identification of lineage projection patterns based on MARCM clones. Dev Biol 384:258–289
Yang JS, Awasaki T, Yu HH, He Y, Ding P, Kao JC, Lee T (2013) Diverse neuronal lineages make stereotyped contributions to the Drosophila locomotor control center, the central complex. J Comp Neurol 15:2645–2662
Young JM, Armstrong JD (2010) Building the central complex in Drosophila: the generation and development of distinct subsets. J Comp Neurol 518:1525–1541
Younossi-Hartenstein A, Nguyen B, Shy D, Hartenstein V (2006) Embryonic origin of the Drosophila brain neuropile. J Comp Neurol 497:981–998
Yu HH, Awasaki T, Schroeder MD, Long F, Yang JS, He Y, Ding P, Kao JC, Wu GY, Peng H, Myers G, Lee T (2013) Clonal development and organization of the adult Drosophila central brain. Curr Biol 23:633–643
Acknowledgements
We thank Dr. J.L.D. Williams for enlightening discussions and Karin Fischer for excellent technical assistance. Financial support was from the Deutsche Forschungsgemeinschaft (BO 1434/3-5) and the Graduate School of Systemic Neuroscience, LMU. Y.L. received support from LMU Munich’s Institutional Strategy “LMUexcellent” within the framework of the German Excellence Initiative. V.H. was supported by NIH grant R01 NS054814.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were performed in accordance with the guidelines for animal welfare as laid down by the Deutsche Forschungsgemeinschaft.
Competing interests
The authors declare that they have no competing interests.
Additional information
Communicated by Claude Desplan
Rights and permissions
About this article
Cite this article
Boyan, G., Liu, Y., Khalsa, S.K. et al. A conserved plan for wiring up the fan-shaped body in the grasshopper and Drosophila . Dev Genes Evol 227, 253–269 (2017). https://doi.org/10.1007/s00427-017-0587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-017-0587-2