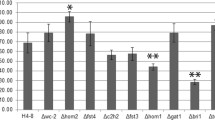
Abstract
Dictyostelium discoideum is a facultative multicellular amoebozoan with cellulose in the stalk and spore coat of its fruiting body as well as in the extracellular matrix of the migrating slug. The organism also harbors a number of cellulase genes. One of them, cbhA, was identified as a candidate cellobiohydrolase gene based on the strong homology of its predicted protein product to fungal cellobiohydrolase I (CBHI). Expression of the cbhA was developmentally regulated, with strong expression in the spores of the mature fruiting body. However, a weak but detectable level of expression was observed in the extracellular matrix at the mound — tipped finger stages, in prestalk O cells, and in the slime sheath of the migrating slug — late culminant stages. A null mutant of the cbhA showed almost normal morphology. However, the developmental timing of the mutant was delayed by 2–4 h. When a c-Myc epitope-tagged CbhA was expressed, it was secreted into the culture medium and was able to bind crystalline cellulose. The CbhA-myc protein was glycosylated, as demonstrated by its ability to bind succinyl concanavalin A-agarose. Moreover, conditioned medium from the cbhA-myc oe strain displayed 4-methylumbelliferyl β-d-cellobioside (4-MUC) digesting activity in Zymograms in which conditioned medium was examined via native-polyacrylamide gel electrophoresis or spotted on an agar plate containing 4-MUC, one of the substrates of cellobiohydrolase. Taken together, these findings indicate that Dictyostelium CbhA is an orthologue of CBH I that is required for a normal rate of development.






Similar content being viewed by others
References
Adney WS, Jeoh T, Beckham GT, Chou Y-C, Baker JO, Michener W, Brunecky R, Himmer ME (2009) Probing the role of N-linked glycans in the stability and activity of fungal cellobiohydrolase by mutational analysis. Cellulose 16:699–709
Baldrian P, Valášková V (2008) Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev 32:501–521
Beckham GT, Bomble YJ, Matthews JF, Taylor CB, Resch MG, Yarbrough JM, Decker SR, Bu L, Zhao X, McCabe C, Wholert J, Bergenstråhle M, Brady JW, Adney WS, Himmer ME, Crowley MF (2010) The O-glycosylated linker from the Trichoderma reesei family 7 cellulase is a flexible, disordered protein. Biophys J 99:3773–3781
Bhat MK (2000) Cellulases and related enzymes in biotechnology. Biotechnol Adv 18:355–383
Birch PR (1998) Targeted differential display of abundantly expressed sequences from the basidiomycete Phanerochaete chrysosporium which contain regions coding for fungal cellulose-binding domains. Curr Genet 33:70–76
Bishop JD, Moon BC, Harrow F, Ratner D, Gomer RH, Dottin RP, Brazill DT (2002) A second UDP-glucose pyrophosphorylase is required for differentiation and development in Dictyostelium discoideum. J Biol Chem 277:32430–32437
Blanton RL (1993) Prestalk cells in monolayer cultures exhibit two distinct modes of cellulose synthesis during stalk cell differentiation in Dictyostelium. Development 119:703–710
Blanton RL, Fuller D, Iranfar N, Grimson MJ, Loomis WF (2000) The cellulose synthase gene of Dictyostelium. Proc Natl Acad USA 97:2391–2396
Blume JE, Ennis HL (1991) A Dictyostelium discoideum cellulase is a member of a spore germination-specific gene family. J Biol Chem 256:15432–15437
Boschker HTS, Cappenberg TE (1994) A sensitive method using 4-methylumbelliferyl-β-cellosiose as a substrate to measure (1,4)-β-glucanase activity in sediments. Appl Environ Microbiol 60:3592–3596
Brown NH (2011) Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb Perspect Biol 3:a005082
Darley CP, Li Y, Schaap P, McQueen-Mason SJ (2003) Expression of a family of expansin-like proteins during the development of Dictyostelium discoideum. FEBS Lett 546:416–418
Dimond RL, Farnsworth PA, Loomis WL (1976) Isolation and characterization of mutations affecting UDPG pyrophosphorylase activity in Dictyostelium discoideum. Dev Biol 50:169–181
Eichinger L, Pachebat JA, Glockner G, Rajandream MA, Sucgang R, Berriman M, Song J, Olsen R, Szafranski K, Xu Q, Tunggal B, Kummerfeld S, Madera M, Konfortov BA, Rivero F, Bankier AT, Lehmann R, Hamlin N, Davies R, Gaudet P, Fey P, Pilcher K, Chen G, Saunders D, Sodergren E, Davis P, Kerhornou A, Ni X, Hall N, Anjard C, Hemphill L, Bason N, Farbrother P, Desany B, Just E, Morio T, Rost R, Churcher C, Cooper J, Haydock S, van Driessche N, Cronin A, Goodhead I, Muzny D, Mourier T, Pain A, Lu M, Harper D, Lindsay R, Hauser H, James K, Quiles M, Madan Babu M, Saito T, Buchrieser C, Wardroper A, Felder M, Thangavelu M, Johnson D, Knights A, Loulseged H, Mungall K, Oliver K, Price C, Quail MA, Urushihara H, Hernandez J, Rabbinowitsch E, Steffen D, Sanders M, Ma J, Kohara Y, Sharp S, Simmonds M, Spiegler S, Tivey A, Sugano S, White B, Walker D, Woodward J, Winckler T, Tanaka Y, Shaulsky G, Schleicher M, Weinstock G, Rosenthal A, Cox EC, Chisholm RL, Gibbs R, Loomis WF, Platzer M, Kay RR, Williams J, Dear PH, Noegel AA, Barrell B, Kuspa A (2005) The genome of the social amoeba Dictyostelium discoideum. Nature 435:43–57
Freeze H, Loomis WF (1977) The role of the fibrillar component of the surface sheath in the morphogenesis of Dictyostelium discoideum. Dev Biol 56:184–194
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147:992–1009
Gao L, Gao F, Wang L, Geng C, Chi L, Zhao J, Qu Y (2012) N-glycoform diversity of cellobiohydrolase I from Penicillium decumbens and synergism of nonhydrolytic glycoform in cellulose degradation. J Biol Chem 287:15906–15915
Gilbert HJ, Hall J, Hazlewood GP, Ferreira LMA (1990) The N-terminal region of an endoglucanase from Pseudomonas fluorencens subspecies cellulosa constitutes a cellulose-binding domain that is distinct from the catalytic centre. Mol Microbiol 4:759–767
Giorda R, Ohmachi T, Shaw DR, Ennis HL (1990) A shared internal threonine–glutamic acid–proline repeat defines a family of Dictyostelium discoideum spore germination specific proteins. Biochemistry 29:7264–7269
Grimson MJ, Haigler CH, Blanton RL (1996) Cellulose microfibrils, cell motility, and plasma membrane protein organization change in parallel during culmination in Dictyostelium discoideum. J Cell Sci 109:3079–3087
Harwood AJ, Drury L (1990) New vectors for expression of the E. coli lacZ gene in Dictyostelium. Nucleic Acids Res 18:4292
Jeoh T, Michener W, Himmer ME, Adney WS (2008) Implications of cellobiohydrolase glycosylation for use in biomass conversion. Biotechnol Biofuels 1:10
Kawata T (2011) STAT signaling in Dictyostelium development. Develop Growth Differ 53:548–557
Kim HY, Nelson CM (2012) Extracellular matrix and cytoskeletal dynamics during morphogenesis. Organogenesis 8:56–64
King AJ, Cragg SM, Li Y, Dymond J, Guille MJ, Bowles DJ, Bruce NC, Graham IA, McQueen-Mason SJ (2010) Molecular insight into lignocellulose digestion by a marine isopod in the absence of gut microbes. Proc Natl Acad USA 107:5345–5350
Labouesse M (2012) Role of the extracellular matrix in epithelial morphogenesis. A view from C. elegans. Organogenesis 8:65–70
Linder M, Teeri T (1996) The cellulose-binding domain of the major cellobiohydrolase of Trichoderma reesei exhibits true reversibility and a high exchange rate on crystalline cellulose. Proc Natl Acad USA 93:12251–12255
Loomis WF (1972) Role of the surface sheath in the control of morphogenesis in Dictyostelium discoideum. Nat New Biol 240:6–9
McGuire V, Alexander S (1996) PsB multiprotein complex of Dictyostelium discoideum. Demonstration of cellulose binding activity and order of protein subunit assembly. J Biol Chem 271:14596–14603
Ogasawara S, Shimada N, Kawata T (2009) Role of an expansin-like molecule in Dictyostelium morphogenesis and regulation of its gene expression by the signal transducer and activator of transcription protein Dd-STATa. Develop Growth Differ 51:109–122
Ramalingam R, Ennis HL (1997) Characterization of the Dictyostelium disicoideum cellulose-binding protein CelB and regulation of gene expression. J Biol Chem 272:26166–26172
Ramalingam R, Blume JE, Ennis HL (1992) The Dictyostelium discoideum spore germination-specific cellulase is organized into functional domains. J Bacteriol 174:7834–7837
Rosness PA (1968) Cellulolytic enzymes during morphogenesis in Dictyostelium discoideum. J Bacteriol 96:639–645
Shimada N, Kawata T (2007) Evidence that noncoding RNA dutA is a multicopy suppressor of Dictyostelium STAT protein, Dd-STATa. Eukaryot Cell 6:1030–1040
Shimada N, Nishio K, Maeda M, Urushihara H, Kawata T (2004a) Extracellular matrix family proteins that are potential targets of Dd-STATa in Dictyostelium discoideum. J Plant Res 117:345–353
Shimada N, Maeda M, Urushihara H, Kawata T (2004b) Identification of new modes of Dd-STATa regulation of gene expression in Dictyostelium by in situ hybridisation. Int J Dev Biol 48:679–682
Shimada N, Maruo T, Maeda M, Urushihara H, Kawata T (2005) Evidence that the Dictyostelium STAT protein Dd-STATa plays a role in the differentiation of inner basal disc cells and identification of a promoter element essential for expression in these cells. Differentiation 73:50–60
Teeri TT (1997) Crystalline cellulose degradation: new insight into the function of cellobiohydrolases. Trends Biotechnol 15:160–167
Teeri TT, Koivula A, Linder M, Wohlfarhrt G, Divne C, Jones TA (1998) Trichoderma reesei cellobiohydrolases: why so efficient on crystalline cellulose? Biochem Soc Trans 26:173–178
Tuohy MG, Walsh DJ, Murray PG, Claeyssens M, Cuffe MM, Savage AV, Coughlan MP (2002) Kinetic parameters and mode pH action of the cellobiohydrolases produced by Talaromyces emersonii. Biochim Biophys Acta 1596:366–380
Wang Y, Slade M, Gooley AA, Atwell BJ, Williams KL (2001) Cellulose-binding modules from extracellular matrix proteins of Dictyostelium discoideum stalk and sheath. Eur J Biochem 268:4334–4345
Zhang J, Zhang Y, Zhong Y, Qu Y, Wang T (2012) Ras GTPases modulate morphogenesis, sporulation and cellulase gene expression in the cellulolytic fungus Trichoderma reesei. PLoS One 7:e48786
Acknowledgments
This work was supported by a Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) to T. Kawata (no. 2150230, 24510307), and a Faculty of Science Special Grant for Promoting Scientific Research at Toho University to T. Kawata (301–12). We thank Dr. Tamao Saito, Sophia University, Japan for her helpful comments on the manuscript. We are grateful to Dr. Margaret K. Nelson, Allegheny College, PA, USA, for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Sureshkumar Balasubramanian
Rights and permissions
About this article
Cite this article
Kunii, M., Yasuno, M., Shindo, Y. et al. A Dictyostelium cellobiohydrolase orthologue that affects developmental timing. Dev Genes Evol 224, 25–35 (2014). https://doi.org/10.1007/s00427-013-0460-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-013-0460-x