Abstract
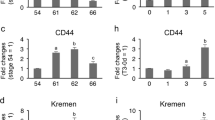
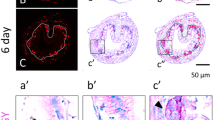
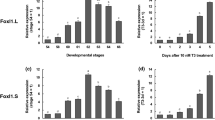
In the Xenopus laevis intestine during metamorphosis, which is triggered by thyroid hormone (TH), the adult epithelium develops and replaces the larval one undergoing apoptosis. We have previously shown that progenitor/stem cells of the adult epithelium originate from some differentiated larval epithelial cells. To investigate molecular mechanisms underlying larval epithelial dedifferentiation into the adult progenitor/stem cells, we here focused on nuclear lamin A (LA) and lamin LIII (LIII), whose expression is generally known to be correlated with the state of cell differentiation. We analyzed the spatiotemporal expression of LA and LIII during X. laevis intestinal remodeling by reverse transcription PCR, Western blotting, and immunohistochemistry. At the onset of natural metamorphosis, when the adult epithelial progenitor cells appear as small islets, the expression of LA is down-regulated, but that of LIII is up-regulated only in the islets. Then, as the adult progenitor cells differentiate, the expression of LA is up-regulated, whereas that of LIII is down-regulated in the adult cells. As multiple intestinal folds form, adult epithelial cells positive for LIII become restricted only to the troughs of the folds. In addition, we have shown that TH up- or down-regulates the expression of these lamins in the premetamorphic intestine as during natural metamorphosis. These results indicate that TH-regulated expression of LA and LIII closely correlates with dedifferentiation of the epithelial cells in the X. laevis intestine, suggesting the involvement of the lamins in the process of dedifferentiation during amphibian metamorphosis.




Similar content being viewed by others
References
Alberio R, Johnson AD, Stick R, Campbell KH (2005) Differential nuclear remodeling of mammalian somatic cells by Xenopus laevis oocyte and egg cytoplasm. Exp Cell Res 307(1):131–141. doi:10.1016/j.yexcr.2005.02.028
Benavente R, Krohne G, Franke WW (1985) Cell type-specific expression of nuclear lamina proteins during development of Xenopus laevis. Cell 41(1):177–190. doi:0092-8674(85)90072-8
Bru T, Clarke C, McGrew MJ, Sang HM, Wilmut I, Blow JJ (2008) Rapid induction of pluripotency genes after exposure of human somatic cells to mouse ES cell extracts. Exp Cell Res 314(14):2634–2642. doi:10.1016/j.yexcr.2008.05.009
Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM (2007) Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature 450(7169):497–502. doi:10.1038/nature06357
Cheng H, Bjerknes M (1985) Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat Rec 211(4):420–426
Constantinescu D, Gray HL, Sammak PJ, Schatten GP, Csoka AB (2006) Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24(1):177–185. doi:10.1634/stemcells.2004-0159
Dechat T, Pfleghaar K, Sengupta K, Shimi T, Shumaker DK, Solimando L, Goldman RD (2008) Nuclear lamins: major factors in the structural organization and function of the nucleus and chromatin. Genes Dev 22(7):832–853. doi:10.1101/gad.1652708
Dodd MHI, Dodd JM (1976) The biology of metamorphosis. In: Lofts B (ed) Physiology of amphibia, vol 3. Academic, New York, pp 467–599
Doring V, Stick R (1990) Gene structure of nuclear lamin LIII of Xenopus laevis; a model for the evolution of IF proteins from a lamin-like ancestor. EMBO J 9(12):4073–4081
Gruenbaum Y, Goldman RD, Meyuhas R, Mills E, Margalit A, Fridkin A, Dayani Y, Prokocimer M, Enosh A (2003) The nuclear lamina and its functions in the nucleus. Int Rev Cytol 226:1–62
Hasebe T, Hartman R, Matsuda H, Shi Y-B (2006) Spatial and temporal expression profiles suggest the involvement of gelatinase A and membrane type 1 matrix metalloproteinase in amphibian metamorphosis. Cell Tissue Res 324(1):105–116. doi:10.1007/s00441-005-0099-7
Hasebe T, Hartman R, Fu L, Amano T, Shi Y-B (2007a) Evidence for a cooperative role of gelatinase A and membrane type-1 matrix metalloproteinase during Xenopus laevis development. Mech Dev 124(1):11–22. doi:10.1016/j.mod.2006.09.001
Hasebe T, Kajita M, Fujimoto K, Yaoita Y, Ishizuya-Oka A (2007b) Expression profiles of the duplicated matrix metalloproteinase-9 genes suggest their different roles in apoptosis of larval intestinal epithelial cells during Xenopus laevis metamorphosis. Dev Dyn 236(8):2338–2345. doi:10.1002/dvdy.21252
Hasebe T, Buchholz DR, Shi Y-B, Ishizuya-Oka A (2011) Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells 29(1):154–161. doi:10.1002/stem.560
Hourdry J, Dauca M (1977) Cytological and cytochemical changes in the intestinal epithelium during anuran metamorphosis. Int Rev Cytol (Supple) 5:337–385
Ishizuya-Oka A, Shi Y-B (2007) Regulation of adult intestinal epithelial stem cell development by thyroid hormone during Xenopus laevis metamorphosis. Dev Dyn 236(12):3358–3368
Ishizuya-Oka A, Ueda S (1996) Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res 286(3):467–476
Ishizuya-Oka A, Ueda S, Inokuchi T, Amano T, Damjanovski S, Stolow M, Shi Y-B (2001) Thyroid hormone-induced expression of sonic hedgehog correlates with adult epithelial development during remodeling of the Xenopus stomach and intestine. Differentiation 69(1):27–37
Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi Y-B (2009) Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. FASEB J 23(8):2568–2575. doi:10.1096/fj.08-128124
Kikuyama S, Kawamura K, Tanaka S, Yamamoto K (1993) Aspects of amphibian metamorphosis: hormonal control. Int Rev Cytol 145:105–148
Kordylewski L (1983) Light and electron microscopic observations of the development of intestinal musculature in Xenopus. Z Mikrosk Anat Forsch 97(4):719–734
Lourim D, Kempf A, Krohne G (1996) Characterization and quantitation of three B-type lamins in Xenopus oocytes and eggs: increase of lamin LI protein synthesis during meiotic maturation. J Cell Sci 109(Pt 7):1775–1785
Madara JL, Trier JS (1994) Functional morphology of the mucosa of the small intestine. In: LR J (ed) Physiology of the gastrointestinal tract, 3rd edn. Raven, New York, pp 1577–1622
Marshall JA, Dixon KE (1978) Cell specialization in the epithelium of the small intestine of feeding Xenopus laevis tadpoles. J Anat 126(1):133–144
McAvoy JW, Dixon KE (1977) Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool 202:129–138
Mitalipov SM, Zhou Q, Byrne JA, Ji WZ, Norgren RB, Wolf DP (2007) Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum Reprod 22(8):2232–2242. doi:10.1093/humrep/dem136
Miyamoto K, Furusawa T, Ohnuki M, Goel S, Tokunaga T, Minami N, Yamada M, Ohsumi K, Imai H (2007) Reprogramming events of mammalian somatic cells induced by Xenopus laevis egg extracts. Mol Reprod Dev 74(10):1268–1277. doi:10.1002/mrd.20691
Moreira PN, Robl JM, Collas P (2003) Architectural defects in pronuclei of mouse nuclear transplant embryos. J Cell Sci 116(Pt 18):3713–3720. doi:10.1242/jcs.00692
Mukhi S, Mao J, Brown DD (2008) Remodeling the exocrine pancreas at metamorphosis in Xenopus laevis. Proc Natl Acad Sci USA 105(26):8962–8967. doi:10.1073/pnas.0803569105
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (Daudin). Garland, New York
Peter M, Nigg EA (1991) Ectopic expression of an A-type lamin does not interfere with differentiation of lamin A-negative embryonal carcinoma cells. J Cell Sci 100(Pt 3):589–598
Quaroni A, Calnek D, Quaroni E, Chandler JS (1991) Keratin expression in rat intestinal crypt and villus cells. Analysis with a panel of monoclonal antibodies. J Biol Chem 266(18):11923–11931
Rankin J, Ellard S (2006) The laminopathies: a clinical review. Clin Genet 70(4):261–274. doi:10.1111/j.1399-0004.2006.00677.x
Rankin SA, Hasebe T, Zorn AM, Buchholz DR (2009) Improved cre reporter transgenic Xenopus. Dev Dyn 238(9):2401–2408. doi:10.1002/dvdy.22043
Rasband WS (1997–2011). Image J. U.S. National Institutes of Health, Bethesda, MD, USA. http://imagej.nih.gov/ij/
Rober RA, Weber K, Osborn M (1989) Differential timing of nuclear lamin A/C expression in the various organs of the mouse embryo and the young animal: a developmental study. Development 105(2):365–378
Shevelyov YY, Lavrov SA, Mikhaylova LM, Nurminsky ID, Kulathinal RJ, Egorova KS, Rozovsky YM, Nurminsky DI (2009) The B-type lamin is required for somatic repression of testis-specific gene clusters. Proc Natl Acad Sci USA 106(9):3282–3287. doi:10.1073/pnas.0811933106
Shi Y-B (1999) Amphibian metamorphosis: from morphology to molecular biology. Wiley, New York
Shi Y-B, Ishizuya-Oka A (2001) Thyroid hormone regulation of apoptotic tissue remodeling: implications from molecular analysis of amphibian metamorphosis. Prog Nucleic Acid Res Mol Biol 65:53–100
Shi Y-B, Liang VC (1994) Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. Biochim Biophys Acta 1217(2):227–228
Stewart C, Burke B (1987) Teratocarcinoma stem cells and early mouse embryos contain only a single major lamin polypeptide closely resembling lamin B. Cell 51(3):383–392. doi:0092-8674(87)90634-9 [pii]
Stick R, Hausen P (1985) Changes in the nuclear lamina composition during early development of Xenopus laevis. Cell 41(1):191–200. doi:0092-8674(85)90073-X
Tata JR (1998) Amphibian metamorphosis as a model for studying the developmental actions of thyroid hormone. Cell Res 8(4):259–272
Wolin SL, Krohne G, Kirschner MW (1987) A new lamin in Xenopus somatic tissues displays strong homology to human lamin A. EMBO J 6(12):3809–3818
Zimek A, Weber K (2005) Terrestrial vertebrates have two keratin gene clusters; striking differences in teleost fish. Eur J Cell Biol 84(6):623–635
Acknowledgments
We would like to thank Drs. Masakazu Fujiwara (Nippon Medical School), Itaru Hasunuma (Waseda Univ), and Kosuke Kawamura (Waseda Univ) for providing us technical information. This work was supported in part by JSPS Grants-in-Aid for Scientific Research (C) to A. I.-O (grant no. 20570060).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Hollemann
Rights and permissions
About this article
Cite this article
Hasebe, T., Kajita, M., Iwabuchi, M. et al. Thyroid hormone-regulated expression of nuclear lamins correlates with dedifferentiation of intestinal epithelial cells during Xenopus laevis metamorphosis. Dev Genes Evol 221, 199–208 (2011). https://doi.org/10.1007/s00427-011-0371-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-011-0371-7