Abstract
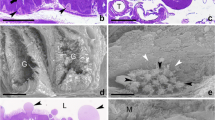
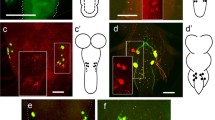
The Drosophila larval and adult midguts are derived from two populations of endodermal progenitors that separate from each other in the early embryo. As larval midgut cells differentiate into an epithelial layer, adult midgut progenitors (AMPs) remain as small clusters of proliferating, undifferentiated cells attached to the basal surface of the larval gut epithelium. During the first few hours of metamorphosis, AMPs merge into a continuous epithelial tube that overgrows the larval layer and differentiates into the adult midgut; at the same time, the larval midgut degenerates. As shown in this paper, there is a second, transient pupal midgut that develops from the AMPs at the beginning of metamorphosis and that intercalates between the adult and larval midgut epithelia. Cells of the transient pupal midgut form a multilayered tube that exhibits signs of differentiation, in the form of septate junctions and rudimentary apical microvilli. Some cells of the pupal midgut develop as endocrine cells. The pupal midgut remains closely attached to the degenerating larval midgut cells. Along with these cells, pupal midgut cells are sequestered into the lumen where they form the compact “yellow body.” The formation of a pupal midgut has been reported from several other species and may represent a general feature of intestinal metamorphosis in insects.







Similar content being viewed by others
References
Affolter M, Walldorf U, Kloter U, Schier AF, Gehring WJ (1993) Regional repression of a Drosophila POU box gene in the endoderm involves inductive interactions between germ layers. Development 117:1199–1210
Andries JC (1970) Activité des nids de régénération de l'intestin moyen de la larve d'Aeschna cyanea au cours d'un cycle digestif. J Ins Phys 16:1961–1973
Andries JC (1979) Junctional structures in the metamorphosing midgut of Aeshna cyanea (Insecta, Odonata). Cell Tissue Res 202:9–15
Ashburner M (1989) Drosophila: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Bach EA, Ekas LA, Ayala-Camargo A, Flaherty MS, Lee H, Perrimon N, Baeg GH (2007) GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr Patterns 7:323–331
Baldwin KM, Hakim RS (1987) Change of form of septate and gap junctions during development of the insect midgut. Tissue Cell 19:549–558
Bodenstein D (1950) The postembryonic development of Drosophila. In: Demerec M (ed) Biology of Drosophila. Wiley, New York, pp 275–367
Campos-Ortega JA, Hartenstein V (1985) The embryonic development of Drosophila melanogaster. Springer, Berlin
Crossley AC (1985) Nephrocytes and pericardial cells. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry, and pharmacology, vol 3. Pergamon, Oxford
Denton D, Shravage B, Simin R, Mills K, Berry DL, Baehrecke EH, Kumar S (2009) Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr Biol 19:1741–1746
Denton D, Shravage B, Simin R, Baehrecke EH, Kumar S (2010) Larval midgut destruction in Drosophila: not dependent on caspases but suppressed by the loss of autophagy. Autophagy 6:163–165
Gallus L, Ferrando S, Bottaro M, Diaspro A, Girosi L, Faimali M, Ramoino P, Tagliafierro G (2009) Presence and distribution of FMRFamide-like immunoreactivity in the cyprid of the barnacle Balanus amphitrite (Cirripedia, Crustacea). Microsc Res Tech 72:101–109
Grigorian M, Mandal L, Hartenstein V (2011) Hematopoiesis at the onset of metamorphosis: terminal differentiation and dissociation of the Drosophila lymph gland. Dev Genes Evol (in press)
Hakim RS, Baldwin K, Smagghe G (2010) Regulation of midgut growth, development, and metamorphosis. Annu Rev Entomol 55:593–608
Hartenstein V, Jan YN (1992) Studying Drosophila embryogenesis with P-lacZ enhancer trap lines. Wilhelm Roux Arch Dev Biol 201:194–220
Haselton AT, Yin CM, Stoffolano JG (2008) FMRFamide-like immunoreactivity in the central nervous system and alimentary tract of the non-hematophagous blow fly, Phormia regina, and the hematophagous horse fly, Tabanus nigrovittatus. J Insect Sci 8:1–17
Jiang H, Edgar BA (2009) EGFR signaling regulates the proliferation of Drosophila adult midgut progenitors. Development 136:483–493
Jirikowski G, Kreissl S, Richter S, Wolff C (2010) Muscle development in the marbled crayfish—insights from an emerging model organism (Crustacea, Malacostraca, Decapoda). Dev Genes Evol 220:89–105
Jung SH, Evans CJ, Uemura C, Banerjee U (2005) The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132:2521–2533
Kathuria OP (1971) Metamorphosis of the midgut of a six-spotted ladybird beetle, Chilomenes sexmaculata (Coleoptera: Coccinellidae). Int J Insect Morphol Embryol 1:87–93
Krajniak KG, Greenberg MJ (1992) The localization of FMRFamide in the nervous and somatic tissues of Nereis virens and its effects upon the isolated esophagus. Comp Biochem Physiol C 101:93–100
Kwok R, Chung D, Brugge VT, Orchard I (2005) The distribution and activity of tachykinin-related peptides in the blood-feeding bug, Rhodnius prolixus. Peptides 26:43–51
Lee CY, Cooksey BA, Baehrecke EH (2002) Steroid regulation of midgut cell death during Drosophila development. Dev Biol 250:101–111
Lehane MJ (1998) The midgut. In: Harrison FW, Locke M (eds) Microscopic anatomy of invertebrates. Wiley Liss, New York, pp 725–746
Liu JL, Murphy C, Buszczak M, Clatterbuck S, Goodman R, Gall JG (2006) The Drosophila melanogaster Cajal body. J Cell Biol 172:875–884
Martoja Rm, Ballan-Dufrancais C (1984) The ultrastructure of the digestive and excretory organs. In: King RC, Akai H (eds) Insect ultrastructure, vol 2. Plenum Press, New York, pp 199–268
Mathur D, Bost A, Driver I, Ohlstein B (2010) A transient niche regulates the specification of Drosophila intestinal stem cells. Science 327:210–213
Micchelli CA, Perrimon N (2006) Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439:475–479
Nakanishi N, Hartenstein V, Jacobs DK (2009) Development of the rhopalial nervous system in Aurelia sp. 1 (Cnidaria, Scyphozoa). Dev Genes Evol 219:301–317
Noirot-Timothee C, Noirot C (1980) Septate and scalariform junctions in arthropods. Int Rev Cytol 63:97–140
Ohlstein B, Spradling A (2006) The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439:470–474
Patel NH, Snow PM, Goodman CS (1987) Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell 48:975–988
Peifer M (1993) The product of the Drosophila segment polarity gene armadillo is part of a multi-protein complex resembling the vertebrate adherens junction. J Cell Sci 105:993–1000
Robertson CW (1936) Metamorphosis of Drosophila melanogaster, including an accurately timed account of the principal morphological changes. J Morphol 59:351–399
Satake H, Kawada T (2006) Overview of the primary structure, tissue-distribution, and functions of tachykinins and their receptors. Curr Drug Targets 7:963–974
Struhl G, Basler K (1993) Organizing activity of wingless protein in Drosophila. Cell 72:527–540
Takashima S, Adams K, Ortiz P, Ying C, Moridzadeh, R, Younossi-Hartenstein A, Hartenstein V (2011) Development of the Drosophila entero-endocrine lineage and its specification by the Notch signaling pathway. Dev Biol. doi:10.1016/j.ydbio.2011.01.039
Tepass U, Hartenstein V (1994a) Epithelium formation in the Drosophila midgut depends on the interaction of endoderm and mesoderm. Development 120:579–590
Tepass U, Hartenstein V (1994b) The development of cellular junctions in the Drosophila embryo. Dev Biol 161:563–596
Tepass U, Hartenstein V (1995) Neurogenic and proneural genes control cell fate specification in the Drosophila endoderm. Development 121:393–405
Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Török T, Hartenstein V (1996) shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Dev 10:672–685
Uemura T, Oda H, Kraut R, Hayashi S, Kotaoka Y, Takeichi M (1996) Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev 10:659–671
Uwo MF, Ui-Tei K, Park P, Takeda M (2002) Replacement of midgut epithelium in the greater wax moth, Galleria mellonela, during larval-pupal moult. Cell Tissue Res 308:319–331
Veenstra JA, Agricola HJ, Sellami A (2008) Regulatory peptides in fruit fly midgut. Cell Tissue Res 334:499–516
Yeo SL, Lloyd A, Kozak K, Dinh A, Dick T, Yang X, Sakonju S, Chia W (1995) On the functional overlap between two Drosophila POU homeo domain genes and the cell fate specification of a CNS neural precursor. Genes Dev 9:1223–1236
Acknowledgments
We thank Dr. Dick Nässel for providing the anti-Tachykinin antibody, the Bloomington Stock Center (Indiana University) and National Institute of Genetics (Japan) for the fly stocks, and the Developmental Studies Hybridoma Bank (University of Iowa) for the antibodies. We also thank Marianne Ciluffo of the BRI electron microscopy facility for help with tissue sectioning and members of the Hartenstein lab for helpful discussion. This work was supported by NIH/1 R01 GM087373 to V.H.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Desplan
Rights and permissions
About this article
Cite this article
Takashima, S., Younossi-Hartenstein, A., Ortiz, P.A. et al. A novel tissue in an established model system: the Drosophila pupal midgut. Dev Genes Evol 221, 69–81 (2011). https://doi.org/10.1007/s00427-011-0360-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-011-0360-x