Abstract
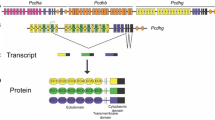
Protocadherins are cadherin-like molecules with adhesive and signaling functions, in particular, during neuronal development. Large protocadherin (Pcdh) gene clusters are present in the genome of vertebrates. In the zebrafish, two Pcdh clusters are found on chromosomes 10 (DrPcdh1) and 14 (DrPcdh2), each divided into subclusters of DrPcdhα and DrPcdhγ family genes. In total, about 100 different DrPcdh molecules are predicted. We have analyzed the expression of the four DrPcdh subclusters and find that DrPcdh transcripts are upregulated in the developing zebrafish nervous system. In the adult fish brain, all four DrPcdh clusters are expressed in differentiated neurons, in particular, in the thalamic nuclei, tectum, and cerebellum. We show that expression patterns grossly overlap for each cluster but with regional differences and variations in strength of expression. Strikingly, the DrPcdh2γ cluster, distinct from the three other clusters, is also expressed in neuronal precursor cells and ependymal cells of the embryonic and adult nervous system, as well as in specific non-neuronal epithelia. Antibodies to a conserved motif in the constant region of DrPcdh2γ stain fiber tracts and neuropil of the zebrafish brain and cell–cell junctions in epithelia. Our results indicate that multiple DrPcdhs of the different clusters are expressed in differentiated zebrafish neurons, suggesting evolutionarily conserved functions of protocadherin clusters in cell adhesion and signaling. In addition, DrPcdh2γ may exert more specific roles in neuronal precursor and non-neural epithelial cells, which have not yet been described for mammalian Pcdhγ. Thus, our findings in zebrafish open new perspectives to examine these functions in other vertebrate model organisms.







Similar content being viewed by others
References
Adolf B, Chapouton P, Lam CS, Topp S, Tannhauser B, Strahle U, Gotz M, Bally-Cuif L (2006) Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev Biol 295:278–293
Babb SG, Barnett J, Doedens AL, Cobb N, Liu Q, Sorkin BC, Yelick PC, Raymond PA, Marrs JA (2001) Zebrafish E-cadherin: expression during early embryogenesis and regulation during brain development. Dev Dyn 221:231–237
Bitzur S, Kam Z, Geiger B (1994) Structure and distribution of N-cadherin in developing zebrafish embryos: morphogenetic effects of ectopic over-expression. Dev Dyn 201:121–136
Down M, Power M, Smith SI, Ralston K, Spanevello M, Burns GF, Boyd AW (2005) Cloning and expression of the large zebrafish protocadherin gene, Fat. Gene Expr Patterns 5:483–490
Ekstrom P, Johnsson CM, Ohlin LM (2001) Ventricular proliferation zones in the brain of an adult teleost fish and their relation to neuromeres and migration (secondary matrix) zones. J Comp Neurol 436:92–110
Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, Hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T (2005) Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet 37:171–176
Frank M, Kemler R (2002) Protocadherins. Curr Opin Cell Biol 14:557–562
Frank M, Ebert M, Shan W, Phillips GR, Arndt K, Colman DR, Kemler R (2005) Differential expression of individual gamma-protocadherins during mouse brain development. Mol Cell Neurosci 29:603–616
Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M (2006) Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol 295:263–277
Haas IG, Frank M, Veron N, Kemler R (2005) Presenilin-dependent processing and nuclear function of gamma-protocadherins. J Biol Chem 280:9313–9319
Hirano S, Wang X, Suzuki ST (2002) Restricted expression of protocadherin 2A in the developing mouse brain. Brain Res Mol Brain Res 98:119–123
Hirano S, Suzuki ST, Redies C (2003) The cadherin superfamily in neural development: diversity, function and interaction with other molecules. Front Biosci 8:d306–d355
Jontes JD, Buchanan J, Smith SJ (2000) Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat Neurosci 3:231–237
Junghans D, Haas IG, Kemler R (2005) Mammalian cadherins and protocadherins: about cell death, synapses and processing. Curr Opin Cell Biol 17:446–452
Kallenbach S, Khantane S, Carroll P, Gayet O, Alonso S, Henderson CE, Dudley K (2003) Changes in subcellular distribution of protocadherin gamma proteins accompany maturation of spinal neurons. J Neurosci Res 72:549–556
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310
Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda R, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T (1998) Diversity revealed by a novel family of cadherins expressed in neurons at a synaptic complex. Neuron 20:1137–1151
Koudijs MJ, den Broeder MJ, Keijser A, Wienholds E, Houwing S, van Rooijen EMH, Geisler R, van Eden FJM (2005) The zebrafish mutants dre, uki and lep encode negative regulators of the hedgehog signaling pathway. PLOS Genet 1(2):e19
Lele Z, Folchert A, Concha M, Rauch GJ, Geisler R, Rosa F, Wilson SW, Hammerschmidt M, Bally-Cuif L (2002) Parachute/N-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development 129:3281–3294
Liu Q, Sanborn KL, Cobb N, Raymond PA, Marrs JA (1999) R-cadherin expression in the developing and adult zebrafish visual system. J Comp Neurol 410:303–319
Meyer A, Schartl M (1999) Gene and genome duplications in vertebrates: the one-to-four (-to-eight in fish) rule and the evolution of novel gene functions. Curr Opin Cell Biol 11:699–704
Mueller T, Wullimann MF (2003) Anatomy of neurogenesis in the early zebrafish brain. Brain Res Dev Brain Res 140:137–155
Murakami T, Hijikata T, Matsukawa M, Ishikawa H, Yorifuji H (2006) Zebrafish protocadherin 10 is involved in paraxial mesoderm development and somitogenesis. Dev Dyn 235:506–514
Nollet F, Kools P, van Roy F (2000) Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol 299:551–572
Noonan JP, Grimwood J, Danke J, Schmutz J, Dickson M, Amemiya CT, Myers RM (2004a) Coelacanth genome sequence reveals the evolutionary history of vertebrate genes. Genome Res 14:2397–2405
Noonan JP, Grimwood J, Schmutz J, Dickson M, Myers RM (2004b) Gene conversion and the evolution of protocadherin gene cluster diversity. Genome Res 14:354–366
Phillips GR, Tanaka H, Frank M, Elste A, Fidler L, Benson DL, Colman DR (2003) Gamma-protocadherins are targeted to subsets of synapses and intracellular organelles in neurons. J Neurosci 23:5096–5104
Redies C, Vanhalst K, Roy F (2005) Delta-protocadherins: unique structures and functions. Cell Mol Life Sci 62:2840–2852
Reiss K, Maretzky T, Haas IG, Schulte M, Ludwig A, Frank M, Saftig P (2006) Regulated ADAM10-dependent ectodomain shedding of gamma-protocadherin C3 modulates cell-cell adhesion. J Biol Chem 281:21735–21744
Rentzsch F, Kramer C, Hammerschmidt M (2003) Specific and conserved roles of TAp73 during zebrafish development. Gene 323:19–30
Schaeren-Wiemers N, Gerfin-Moser A (1993) A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: in situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry 100:431–440
Seiler C, Finger-Baier KC, Rinner O, Makhankov YV, Schwarz H, Neuhauss SC, Nicolson T (2005) Duplicated genes with split functions: independent roles of protocadherin15 orthologues in zebrafish hearing and vision. Development 132:615–623
Tada MN, Senzaki K, Tai Y, Morishita H, Tanaka YZ, Murata Y, Ishii Y, Asakawa S, Shimizu N, Sugino H, Yagi T (2004) Genomic organization and transcripts of the zebrafish protocadherin genes. Gene 340:197–211
Tasic B, Nabholz CE, Baldwin KK, Kim Y, Rueckert EH, Ribich SA, Cramer P, Wu Q, Axel R, Maniatis T (2002) Promoter choice determines splice site selection in protocadherin alpha and gamma pre-mRNA splicing. Mol Cell 10:21–33
Thisse C, Thisse B, Schilling TF, Postlethwait JH (1993) Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 119:1203–1215
Vanhalst K, Kools P, Vanden Eynde E, van Roy F (2001) The human and murine protocadherin-beta one-exon gene families show high evolutionary conservation, despite the difference in gene number. FEBS Lett 495:120–125
Wang X, Su H, Bradley A (2002a) Molecular mechanisms governing Pcdh-gamma gene expression: evidence for a multiple promoter and cis-alternative splicing model. Genes Dev 16:1890–1905
Wang X, Weiner JA, Levi S, Craig AM, Bradley A, Sanes JR (2002b) Gamma protocadherins are required for survival of spinal interneurons. Neuron 36:843–854
Weiner JA, Wang X, Tapia JC, Sanes JR (2005) Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA 102:8–14
Westerfield M (1994) The zebrafish book: a guide for the laboratory use of zebrafish. University of Oregon Press, Eugene, Oregon
Wu Q (2005) Comparative genomics and diversifying selection of the clustered vertebrate protocadherin genes. Genetics 169:2179–2188
Wu Q, Maniatis T (1999) A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell 97:779–790
Wu Q, Zhang T, Cheng JF, Kim Y, Grimwood J, Schmutz J, Dickson M, Noonan JP, Zhang MQ, Myers RM, Maniatis T (2001) Comparative DNA sequence analysis of mouse and human protocadherin gene clusters. Genome Res 11:389–404
Wullimann MF, Knipp S (2000) Proliferation pattern changes in the zebrafish brain from embryonic through early postembryonic stages. Anat Embryol 202:385–400
Wullimann MF, Rupp B, Reichert H (1996) Neuoanatomy of the zebrafish brain: a topological atlas. Birkhäuser, Basel
Zupanc GK, Hinsch K, Gage FH (2005) Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol 488:290–319
Acknowledgments
We are grateful to Dr. Rolf Kemler for his continuous support of this project. We thank Melanie Walter and Volker Haid for excellent technical assistance, Donatus Bönsch for supreme fish care, and Dr. Ingrid Haas and the members of the Hammerschmidt laboratory for many valuable discussions. In addition, we thank Dr. F. van Eeden for providing the probe for PCNA analysis. The contributions of M. Rodriguez-Guzman in the initial phase of the project are acknowledged. This study was done with support of the Max-Planck Society.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Hollemann
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Overview of primers used in RT-PCR and probe generation (DOC 23 kb)
Supplementary Figure S2
DrPcdh1α and DrPcdh2γ transcripts are expressed in epithelia of the gut Detection of DrPcdh1α (a) and DrPcdh2γ (b) transcripts on cross sections of gut epithelia of five day old fish. In the adult stomach epithelium (c-e), very low levels of DrPcdh1α remain (c). DrPcdh1γ transcripts are not expressed in the gut epithelium in of adult fish (d), whereas DrPcdh2γ transcript expression is maintained at high levels in the adult (e). Scale bars are 50 μm (GIF 392 kb).
Rights and permissions
About this article
Cite this article
Bass, T., Ebert, M., Hammerschmidt, M. et al. Differential expression of four protocadherin alpha and gamma clusters in the developing and adult zebrafish: DrPcdh2γ but not DrPcdh1γ is expressed in neuronal precursor cells, ependymal cells and non-neural epithelia. Dev Genes Evol 217, 337–351 (2007). https://doi.org/10.1007/s00427-007-0145-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-007-0145-4