Abstract.
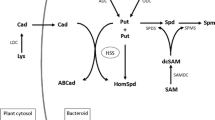
The polyamine spermidine is an essential biosynthetic precursor of pyrrolizidine alkaloids. It provides its aminobutyl group which is transferred to putrescine yielding homospermidine, the specific building block of the necine base moiety of pyrrolizidine alkaloids. The enzymatic formation of spermidine was studied in relation to the unique role of this polyamine as an alkaloid precursor. S-adenosylmethionine decarboxylase (SAMDC, EC 4.1.1.50) and spermidine synthase (SPDS, EC 2.5.1.16) from root cultures of Senecio vulgaris were partially purified and characterized. The SAMDC-catalyzed reaction showed a pH optimum of 7.5, that of SPDS an optimum of 7.7. The K m value of SAMDC for its substrate S-adenosylmethionine (SAM) was 15 μM, while the apparent K m values of SPDS for its substrates decarboxylated SAM (dSAM) and putrescine were 4 μM and 21 μM, respectively. The relative molecular masses of the two enzymes, determined by gel filtration, were 29 000 (SAMDC) and 37 000 (SPDS). Studies with various potential inhibitors revealed, for most inhibitors, profiles that were similar to those established with the respective enzymes from other plant sources. However, putrescine which is not known to be an inhibitor of plant SAMDC, strongly inhibited the enzyme from S. vulgaris roots. Spermidine synthase was sensitive to inhibition by its product spermidine. In the presence of the stationary tissue concentrations of the two polyamines (ca. 0.1 mM each) the activities of SAMDC and SPDS would be inhibited by >80%. The results are discussed in relation to the role of spermidine in primary and secondary metabolism of alkaloid-producing S. vulgaris root cultures.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 15 September 1999 / Accepted 10 December 1999
Rights and permissions
About this article
Cite this article
Graser, G., Hartmann, T. Biosynthesis of spermidine, a direct precursor of pyrrolizidine alkaloids in root cultures of Senecio vulgaris L.. Planta 211, 239–245 (2000). https://doi.org/10.1007/s004250000260
Issue Date:
DOI: https://doi.org/10.1007/s004250000260