Abstract
Main conclusion
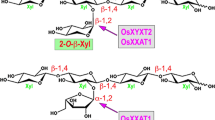
Several pine members of the gymnosperm-specific GT61 clades were demonstrated to be arabinosyltransferases and xylosyltransferases catalyzing the transfer of 2-O-Araf, 3-O-Araf and 2-O-Xyl side chains onto xylooligomer acceptors, indicating their possible involvement in Araf and Xyl substitutions of xylan in pine.
Abstract
Xylan in conifer wood is substituted at O-2 with methylglucuronic acid (MeGlcA) as well as at O-3 with arabinofuranose (Araf), which differs from xylan in dicot wood that is typically decorated with MeGlcA but not Araf. Currently, glycosyltransferases responsible for conifer xylan arabinosylation have not been identified. Here, we investigated the roles of pine glycosyltransferase family 61 (GT61) members in xylan substitutions. Biochemical characterization of four pine wood-associated GT61 members showed that they exhibited three distinct glycosyltransferase activities involved in xylan substitutions. Two of them catalyzed the addition of 2-O-α-Araf or 3-O-α-Araf side chains onto xylooligomer acceptors and thus were named Pinus taeda xylan 2-O-arabinosyltransferase 1 (PtX2AT1) and 3-O-arabinosyltransferase 1 (PtX3AT1), respectively. Two other pine GT61 members were found to be xylan 2-O-xylosyltransferases (PtXYXTs) adding 2-O-β-Xyl side chains onto xylooligomer acceptors. Furthermore, sequential reactions with PtX3AT1 and the PtGUX1 xylan glucuronyltransferase demonstrated that PtX3AT1 could efficiently arabinosylate glucuronic acid (GlcA)-substituted xylooligomers and likewise, PtGUX1 was able to add GlcA side chains onto 3-O-Araf-substituted xylooligomers. Phylogenetic analysis revealed that PtX2AT1, PtX3AT1 and PtXYXTs resided in three gymnosperm-specific GT61 clades that are separated from the grass-expanded GT61 clade harboring xylan 3-O-arabinosyltransferases and 2-O-xylosyltransferases, suggesting that they might have been recruited independently for xylan substitutions in gymnosperms. Together, our findings have established several pine GT61 members as xylan 2-O- and 3-O-arabinosyltransferases and 2-O-xylosyltransferases and they indicate that pine xylan might also be substituted with 2-O-Araf and 2-O-Xyl side chains.







Similar content being viewed by others
Data availability
All data generated during this study are included in this article and its Supplementary Figure files.
References
Anders N, Wilkinson MD, Lovegrove A, Freeman J, Tryfona T, Pellny TK et al (2012) Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc Natl Acad Sci USA 109:989–993
Andersson S-I, Samuelson O, Ishihara M, Shimizu K (1983) Structure of the reducing end-groups in spruce xylan. Carbohydr Res 111:283–288
Biely P, Singh S, Puchart V (2016) Towards enzymatic breakdown of complex plant xylan structures: state of the art. Biotechnol Adv 34:1260–1274
Busse-Wicher M, Li A, Silveira RL, Pereira CS, Tryfona T, Gomes TC et al (2016) Evolution of xylan substitution patterns in gymnosperms and angiosperms: implications for xylan interaction with cellulose. Plant Physiol 171:2418–3241
Evtuguin DV, Tomás JL, Silva AM, Neto CP (2003) Characterization of an acetylated heteroxylan from Eucalyptus globulus Labill. Carbohydr Res 338:597–604
Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (1998) Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281:237–240
Fischer MH, Yu N, Gray GR, Ralph J, Anderson L, Marlett JA (2004) The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohydr Res 339:2009–2017
Haghighat M, Teng Q, Zhong R, Ye Z-H (2016) Evolutionary conservation of xylan biosynthetic genes in Selaginella moellendorffii and Physcomitrella patens. Plant Cell Physiol 57:1707–1719
Hoffmann RA, Leeflang BR, de Barse MM, Kamerling JP, Vliegenthart JF (1991) Characterisation by 1H-n.m.r. spectroscopy of oligosaccharides, derived from arabinoxylans of white endosperm of wheat, that contain the elements ->4)[alpha-L-Araf-(1->3)]-beta-D-Xylp-(1-> or ->4)[alpha-L-Araf-(1->2)][alpha-L-Araf-(1->3)]-beta-D-Xylp-(1->. Carbohydr Res 221:63–81
Höije A, Sandstrom C, Roubroeks JP, Andersson R, Gohil S, Gatenholm P (2006) Evidence of the presence of 2-O-β-d-xylopyranosyl-α-l-arabinofuranose side chains in barley husk arabinoxylan. Carbohydr Res 341:2959–2966
Ishii T, Ichita J, Matsue H, Ono H, Maeda I (2002) Fluorescent labeling of pectic oligosaccharides with 2-aminobenzamide and enzyme assay for pectin. Carbohydr Res 337:1023–1032
Jacobs A, Larsson PT, Dahlman O (2001) Distribution of uronic acids in xylans from various species of soft- and hardwood as determined by MALDI mass spectrometry. Biomacromol 2:979–990
Jensen JK, Busse-Wicher M, Poulsen CP, Fangel JU, Smith PJ, Yang JY et al (2018) Identification of an algal xylan synthase indicates that there is functional orthology between algal and plant cell wall biosynthesis. New Phytol 218:1049–1060
Jiménez-Ortega E, Valenzuela S, Ramírez-Escudero M, Pastor FJ, Sanz-Aparicio J (2020) Structural analysis of the reducing-end xylose-releasing exo-oligoxylanase Rex8A from Paenibacillus barcinonensis BP-23 deciphers its molecular specificity. FEBS J 287:5362–5374
Kulkarni AR, Peña MJ, Avci U, Mazumder K, Urbanowicz BR, Pattathil S et al (2012) The ability of land plants to synthesize glucuronoxylans predates the evolution of tracheophytes. Glycobiology 22:439–451
Lee C, Teng Q, Zhong R, Ye Z-H (2012) Arabidopsis GUX proteins are glucuronyltransferases responsible for the addition of glucuronic acid side chains onto xylan. Plant Cell Physiol 53:1204–1216
Lorenz WW, Yu Y-S, Dean JFD (2010) An improved method of RNA isolation from loblolly pine (P. taeda L.) and other conifer species. J vis Exp 36:1751
Lyczakowski JJ, Yu L, Terrett OM, Fleischmann C, Temple H, Thorlby G et al (2021) Two conifer GUX clades are responsible for distinct glucuronic acid patterns on xylan. New Phytol 231:1720–1733
Martínez-Abad A, Berglund J, Toriz G, Gatenholm P, Henriksson G, Lindström M et al (2017) Regular motifs in xylan modulate molecular flexibility and interactions with cellulose surfaces. Plant Physiol 175:1579–1592
McCleary BV, McKie VA, Draga A, Rooney E, Mangan D, Larkin J (2015) Hydrolysis of wheat flour arabinoxylan, acid-debranched wheat flour arabinoxylan and arabino-xylo-oligosaccharides by β-xylanase, α-l-arabinofuranosidase and β-xylosidase. Carbohydr Res 407:79–96
Mortimer JC, Miles GP, Brown DM, Zhang Z, Segura MP, Weimar T et al (2010) Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci USA 107:17409–17414
Pagny S, Bouissonnie F, Sarkar M, Follet-Gueye ML, Driouich A, Schachter H et al (2003) Structural requirements for Arabidopsis β1,2-xylosyltransferase activity and targeting to the Golgi. Plant J 33:189–203
Peña MJ, Zhong R, Zhou G-K, Richardson EA, O’Neill MA, Darvill AG et al (2007) Arabidopsis irregular xylem8 and irregular xylem9: Implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 19:549–563
Peña MJ, Kulkarni AR, Backe J, Boyd M, O’Neill MA, York WS (2016) Structural diversity of xylans in the cell walls of monocots. Planta 244:589–606
Teleman A, Tenkanen M, Jacobs A, Dahlman O (2002) Characterization of O-acetyl-(4-O-methylglucurono)xylan isolated from birch and beech. Carbohydr Res 337:373–377
Timell TE (1967) Recent progress in the chemistry of wood hemicelluloses. Wood Sci Technol 1:45–70
Verbruggen MA, Spronk BA, Schols HA, Beldman G, Voragen AG, Thomas JR et al (1998) Structures of enzymically derived oligosaccharides from sorghum glucuronoarabinoxylan. Carbohydr Res 306:265–274
Weng J-K, Li X, Stout J, Chapple C (2008) Independent origins of syringyl lignin in vascular plants. Proc Natl Acad Sci USA 105:7887–7892
Weng J-K, Akiyama T, Ralph J, Chapple C (2011) Independent recruitment of an O-methyltransferase for syringyl lignin biosynthesis in Selaginella moellendorffii. Plant Cell 23:2708–2724
Ye Z-H, Zhong R (2022) Cell wall biology of the moss Physcomitrium patens. J Exp Bot 73:4440–4453
Zhong R, Peña MJ, Zhou G-K, Nairn CJ, Wood-Jones A, Richardson EA et al (2005) Arabidopsis Fragile Fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. Plant Cell 17:3390–3408
Zhong R, Cui D, Phillips DR, Ye Z-H (2018) A novel rice xylosyltransferase catalyzes the addition of 2-O-xylosyl side chains onto the xylan backbone. Plant Cell Physiol 59:554–565
Zhong R, Cui D, Ye Z-H (2019) Secondary cell wall biosynthesis. New Phytol 221:1703–1723
Zhong R, Cui D, Phillips DR, Sims NT, Ye Z-H (2021) Functional analysis of GT61 glycosyltransferases from grass species in xylan substitutions. Planta 254:131
Zhong R, Lee C, Cui D, Phillips DR, Adams ER, Jeong HY et al (2022) Identification of xylan arabinosyl 2-O-xylosyltransferases catalyzing the addition of 2-O-xylosyl residue onto arabinosyl side chains of xylan in grass species. Plant J. https://doi.org/10.1111/tpj.15939
Acknowledgements
This work was funded by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences [grant no. DE-FG02-03ER15415]. We thank the UGA Proteomics and Mass Spectrometry Core Facility and the UGA Chemistry NMR Facility for providing the instruments used in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Anastasios Melis.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhong, R., Phillips, D.R. & Ye, ZH. Independent recruitment of glycosyltransferase family 61 members for xylan substitutions in conifers. Planta 256, 70 (2022). https://doi.org/10.1007/s00425-022-03989-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-03989-x