Abstract
Main conclusion
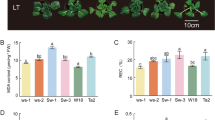
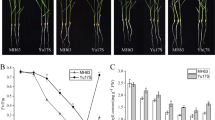
Short-term cold stress can induce the increased expression of key enzyme-encoding genes involved in secondary metabolite synthesis, thereby increasing secondary metabolite concentration.
Abstract
Cold stress is an ecologically limiting factor that strongly affects the physiological and biochemical properties of medicinal plants often resulting in changes of the secondary metabolic process. Ginsenosides are the main active ingredients in medicinal ginseng yet few studies exist on the effect of cold stress on the expression of ginsenosides or the molecular mechanism underlying its regulation. Here, we evaluated the effects of cold stress on the physiological characteristics and secondary metabolism of P. ginseng embryogenic calli. Physiological measurements and RNA-Seq analysis were used to dissect the metabolic and molecular responses of P. ginseng to cold conditions. We found that the dynamic accumulation of ginsenoside and various physiological indicators leads to homogenous adaptation to cold stress. Secondary metabolism of ginseng could be a compensation mechanism to facilitate its adaptation to cold stress. Combined with the changes in the endogenous hormone content, 9-cis-epoxycarotenoid dioxygenase (NCED), zeaxanthin epoxidase (ZEP), and short chain dehydrogenase (SDR) from the abscisic acid (ABA) synthesis pathway were identified as key mediators of this response. Thus, an appropriate degree of cold stress may promote accumulation of ginsenosides. Moreover, 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR2), squalene epoxidase (SE1), squalene synthase (SS), dammarenediol synthase (DS-II), and β-alanine C-28 hydroxylase (CYP716A52v2) should be considered key mediators of the cold stress response and ginsenoside biosynthesis. During industrial production, short-term cold stress should be carried out on ginseng calli to improve the quality of its medicinal materials.
















Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- CYP716A52v2 :
-
β-Alanine C-28 hydroxylase
- DS :
-
Dammarenediol synthase
- HMGR :
-
3-Hydroxy-3-methylglutaryl-CoA reductase
- JA:
-
Jasmonic acid
- MDA:
-
Malondialdehyde
- NCED:
-
9-Cis-epoxycarotenoid dioxygenase
- POD:
-
Peroxidase
- SA:
-
Salicylic acid
- SDR:
-
Short chain dehydrogenase
- SE :
-
Squalene epoxidase
- SS1 :
-
Squalene synthase
- SOD:
-
Superoxide dismutase
- SP:
-
Soluble protein
- SS:
-
Soluble sugars
- ZEP:
-
Zeaxanthin epoxidase
References
Aghdam MS, Asghari M, Khorsandi O, Mohayeji M (2014) Alleviation of postharvest chilling injury of tomato fruit by salicylic acid treatment. J Food Sci Technol 51:2815–2820
Anderson JT, Mitchell-Olds T (2011) Ecological genetics and genomics of plant defenses: evidence and approaches. Funct Ecol 25:312–324
Avanci NC, Luche DD, Goldman GH, Goldman MHS (2010) Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. Genet Mol Res 9:484–505
Ba QS, Zhang GS, Wang JS, Che HX, Liu HZ, Niu N, Ma SC, Wang JW (2013) Relationship between metabolism of reactive oxygen species and chemically induced male sterility in wheat. Can J Plant Sci 93:675–681
Balestrasse KB, Tomaro ML, Batlle A, Noriega GO (2010) The role of 5-aminolevulinic acid in the response to cold stress in soybean plants. Phytochemistry 71:2038–2045
Bostock RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43:545–580
Chen QF, Ya HY, Feng YR, Jiao Z (2014) Expression of the key genes involved in ABA biosynthesis in rice implanted by ion beam. Appl Biochem Biotechnol 173:239–247
Cheng L, Han M, Yang L et al (2018) Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind Crops Prod 122:473–482
Commission CP (2020) Pharmacopoeia of the People’s Republic of China. The medicine science and technology press of China, China
Corbineau F, Gay-Mathieu C, Vinel D, Côme D (2010) Decrease in sunflower (Helianthus annuus) seed viability caused by high temperature as related to energy metabolism, membrane damage and lipid composition. Physiol Plant 116:489–496
Crifò T, Puglisi I, Petrone G et al (2011) Expression analysis in response to low temperature stress in blood oranges: implication of the flavonoid biosynthetic pathway. Gene 476:1–9
Fujita M, Fujita Y, Noutoshi Y et al (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Grabherr MG, Haas BJ, Yassour M et al (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29:644–652
Han JY, Hwang HS, Choi SW et al (2012) Cytochrome P450 CYP716A53v2 catalyzes the formation of protopanaxatriol from protopanaxadiol during ginsenoside biosynthesis in Panax ginseng. Plant Cell Physiol 53:1535–1545
Háznagy-Radnai E, Czigle S, Zupkó I et al (2006) Comparison of antioxidant activity in enzyme-independent system of six Stachys species. Fitoterapia 77:521–524
Jang GY, Kim MY, Lee YJ et al (2018) Influence of organic acids and heat treatment on ginsenoside conversion. J Ginseng Res 42:532–539
Janská A, Marsík P, Zelenková S, Ovesná J (2010) Cold stress and acclimation – what is important for metabolic adjustment? Plant Biol 12:395–405
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162:929–936
Jeong HN, Lee KJ, Jeong JS et al (2016) Comparison of quality and ginsenoside contents in roots of Panax ginseng at various ages in Gangwon. Korea. Planta Med 82(S01):S1–S381
Ji Y, Rao Z, Cui J et al (2012) Ginsenosides extracted from nanoscale Chinese white ginseng enhances anticancer effect. J Nanosci Nanotechnol 12:1–25
Jiang M, Liu J, Quan X et al (2016) Different chilling stresses stimulated the accumulation of different types of different types of ginsenosides in P. ginseng cells. Acta Physiol Plant 210:1–8
Jin J, Kwang KJ, Qi Wu EA (2018) Effects of cold stress on transcripts and metabolites in tartary buckwheat (Fagopyrum tataricum). Environ Exp Bot 155:488–496
Juxin Y, Zhang D, Zhuang J, Huang Y, Mu Y, Lv S (2017) Study on the correlation between gene expression and enzyme activity of seven key enzymes and ginsenoside content in ginseng in over time in Ji’an, China. Int J Mol Sci 18:1–12
Kim KS, Ban YJ (2017) The effect of Korean red ginseng on chronic non-bacterial prostatitis. Planta Med Int Open 4(0S1):20–25
Kim YJ, Lee OR, Oh JY et al (2014) Functional analysis of 3-hydroxy-3-methylglutaryl coenzyme A reductase encoding genes in triterpene saponin-producing ginseng. Plant Physiol 165:373–387
Kim Young-Joo S-D, Sae-Woong Kim KJ-SH, Sohn D (2013) Improvement of erectile function by Korean red ginseng (P. ginseng) in a male rat model of metabolic syndrome. Asian J Androl 15:395–399
Lee S (2018) High-performance liquid chromatography analysis of phytosterols in P. ginseng root grown under different conditions. J Ginseng Res 49:16–20
Lee C, Shim S, Park H, Han K (1980) Mass production method of Korea ginseng (P. ginseng C.A. Meyer) leaf and inhibitory effect of extracts on fat accumulation. Korean J Ginseng Sci 4:55–64
Lee DG, Cho S, Lee J et al (2016) Isolation and identification of a new dammarane-type triterpene saponin from P. ginseng. Planta Med 82:S1–S381
Lee YS, Park H, Lee D et al (2017) Integrated transcriptomic and metabolomic analysis of five Panax ginseng cultivars reveals the dynamics of ginsenoside biosynthesis. Front Plant Sci 8:1048. https://doi.org/10.3389/fpls.2017.01048
Lo’Ay AA, EL-Khateeb AY (2018) Antioxidant enzyme activities and exogenous ascorbic acid treatment of “Williams” banana during long-term cold storage stress. Sci Hortic 234:210–219
Lou Y, Sun H, Li L et al (2017) Characterization and primary functional analysis of a bamboo ZEP gene from Phyllostachys edulis. DNA Cell Biol 36:747–758
Mauch-Mani B, Mauch F (2005) The role of abscisic acid in plant–pathogen interactions. Curr Opin Plant Biol 8:409–414
Megha S, Basu U, Joshi RK, Kav NNV (2018) Physiological studies and genome-wide microRNA profiling of cold-stressed Brassica napus. Plant Physiol Biochem 132:1–17
Mei Z, Bing Y, Ping L (2009) Cloning of 9-cis-epoxycarotenoid dioxygenase (NCED) gene and the role of ABA on fruit ripening. Plant Signal Behav 4:460–463
Murukarthick Jayakodi SLTY (2018) Comparative transcriptome analysis of heat stress responsiveness between two contrasting ginseng cultivars. J Ginseng Res 43:572–579
Oh JY, Kim YJ, Jang MG et al (2014) Investigation of ginsenosides in different tissues after elicitor treatment in Panax ginseng. J Ginseng Res 38:270–277
Patro R, Duggal G, Love MI et al (2017) Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods 14:417–419
Radad K, Gille G, Liu L, Rausch WD (2006) Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci 100:175–186
Rahimi Y, Taleei A, Ranjbar M (2018) Long-term water deficit modulates antioxidant capacity of peppermint (Mentha piperita L.). Sci Hortic 237:36–43
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140
Rogerio AP, Sá-Nunes A, Faccioli LH (2010) The activity of medicinal plants and secondary metabolites on eosinophilic inflammation. Pharmacol Res 62:298–307
Tao J, Li L (2002) Enzymatic regulation of abscisic acid biosynthesis in higher plants. Chin Bull Bot 6:675–683
Thiyagarajan R, Kim SW, Jong-Hwan S-KY, Kim S (2012) Effect of fermented P. ginseng extract (GINST) on oxidative stress and antioxidant activities in major organs of aged rats. Exp Gerontol 47:77–84
Tritsch D, Hemmerlin A, Bach TJ, Rohmer M (2010) Plant isoprenoid biosynthesis via the MEP pathway: in vivo IPP/DMAPP ratio produced by (E)-4-hydroxy-3-methylbut-2-enyl diphosphate reductase in tobacco BY-2 cell cultures. FEBS Lett 584:129–134
Wang J, Li J, Li J, Liu S, Wu X, Li J, Gao W (2016) Transcriptome profiling shows gene regulation patterns in ginsenoside pathway in response to methyl jasmonate in Panax Quinquefolium adventitious root. Sci RepoRts 1–11
Wang B, Zhu J, Pierson E et al (2017) Visualization and analysis of single-cell RNA-seq data by kernel-based similarity learning. Nat Methods 14:414–416
Yang C, Zhang T, Xu M et al (2016) Insights into biosynthetic genes involved in the secondary metabolism of Gardenia jasminoides Ellis using transcriptome sequencing. Biochem Syst Ecol 67:7–16
Yang J, Hu Z, Zhang T, Gu A, Gong T, Zhu P (2018) Progress on the studies of the key enzymes of ginsenoside biosynthesis. Molecules 589:1–12
You J, Liu X, Zhang B et al (2015) Seasonal changes in soil acidity and related properties in ginseng artificial bed soils under a plastic shade. J Ginseng Res 39:81–88
Zhang T (2019) Physiological and ecological response mechanism of P. ginseng and its saponins biosynthesis to low temperature. Jilin Agriculture University, Changchun, China, pp 1–136
Acknowledgements
This work was supported by the major science and technology projects of the Jilin Province (20200504002YY), the National Key Research and Development Programs of China (2019YFC1710700), and the Chinese Agricultural Research System (grant number CARS-21).
Author information
Authors and Affiliations
Contributions
MH and LY conceived and designed the research. TZ performed the experiments and prepared the manuscript; YG completed the experimental data processing; all authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.




425_2020_3535_MOESM5_ESM.png
Supplementary file5 (PNG 175 KB) Fig. S5 Analysis of T_6 vs C_6 differentially expressed gene KEGG enrichment. Fig. S6 Validation of the transcriptome data using qRT-PCR. Vertical bars indicate the mean value ± SD from three independent experiments. *P < 0.05 and **P < 0.01 denote significant differences from the control, respectively.
425_2020_3535_MOESM6_ESM.xlsx
Supplementary file6 (XLSX 10 KB) Table S1 FPKM results of ten randomly selected P. ginseng calli unigenes identified during high-throughput transcriptome sequencing
Rights and permissions
About this article
Cite this article
Zhang, T., Gao, Y., Han, M. et al. Changes in the physiological characteristics of Panax ginseng embryogenic calli and molecular mechanism of ginsenoside biosynthesis under cold stress. Planta 253, 79 (2021). https://doi.org/10.1007/s00425-020-03535-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03535-7