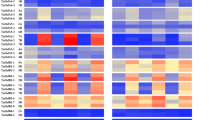
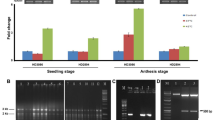
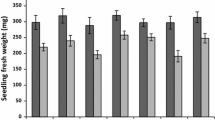
Abstract
Main conclusion
Overexpressing a heat shock factor gene ( TaHsfA6b T) from wheat provides thermotolerance in barley by constitutive expression of heat and other abiotic stress-response genes.
Abstract
Temperature is one of the most crucial abiotic factors defining the yield potential of temperate cereal crops, such as barley. The regulators of heat shock response (HSR), heat stress transcription factors (Hsfs), modulate the transcription level of heat-responsive genes to protect the plants from heat stress. In this study, an Hsf from wheat (TaHsfA6b) is overexpressed in barley for providing thermotolerance. Transgenic barley lines overexpressing TaHsfA6b showed improvement in thermotolerance. The constitutive overexpression of a TaHsfA6b gene upregulated the expression of major heat shock proteins and other abiotic stress-responsive genes. RNA-seq and qRT-PCR analysis confirmed the upregulation of Hsps, chaperonins, DNAJ, LEA protein genes, and genes related to anti-oxidative enzymes in transgenic lines. Excessive generation and accumulation of reactive oxygen species (ROS) occurred in wild-type (WT) plants during heat stress; however, the transgenic lines reflected improved ROS homeostasis mechanisms, showing lesser ROS accumulation under high temperature. No negative phenotypic changes were observed in overexpression lines. These results suggest that TaHsfA6b is a regulator of HSR and its overexpression altered the expression patterns of some main stress-related genes and enhanced the thermotolerance of this cereal crop.







Similar content being viewed by others
Abbreviations
- HS:
-
Heat stress
- HSR:
-
Heat stress response
- HSF:
-
Heat stress transcription factor
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- SOD:
-
Superoxide dismutase
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Alptekin B, Langridge P, Budak H (2017) Abiotic stress miRNomes in the Triticeae. Funct Integr Genomics 17(2–3):145–170
Anjum NA, Sofo A, Scopa A, Roychoudhury A, Gill SS, Iqbal M, Lukatkin AS, Pereira E, Duarte AC, Ahmad I (2015) Lipids and proteins—major targets of oxidative modifications in abiotic stressed plants. Environ Sci Pollut Res Int 22(6):4099–4121
Badawi GH, Yamauchi Y, Kawano N, Tanaka K, Tanaka K (2004) Enhanced tolerance to water deficit and high salt stress by overexpressing superoxide dismutase and ascorbate peroxidase in tobacco chloroplasts. Plant Cell Physiol 45:S230–S230
Baldawi M, Danyluk J, Boucho B, Houde M, Sarhan F (2007) The CBF gene family in hexaploid wheat and its relationship to the phylogenetic complexityof cereal CBFs. Mol Genet Genomics 277:533–554
Baniwal SK, Chan KY, Scharf KD, Nover L (2007) Role of heat stress transcription factor HsfA5 as specific repressor of HsfA4. J Biol Chem 282(6):3605–3613
Banti V, Mafessoni F, Loreti E, Alpi A, Perata P (2010) The heat-inducible transcription factor HsfA2 enhances anoxia tolerance in Arabidopsis. Plant Physiol 152(3):1471–1483
Bharti K, von Koskull-Döring P, Bharti S, Kumar P, Tintschl-Körbitzer A, Treuter E, Nover L (2004) Tomato heat stress transcription factor HsfB1 represents a novel type of general transcription coactivator with a histone-like motif interacting with the plant CREB binding protein ortholog HAC1. Plant Cell 16(6):1521–1535
Brocker C, Vasiliou M, Carpenter S, Carpenter C, Zhang Y, Wang X, Kotchoni SO, Wood AJ, Kirch HH, Kopečný D, Nebert DW, Vasiliou V (2013) Aldehyde dehydrogenase (ALDH) superfamily in plants: gene nomenclature and comparative genomics. Planta 237(1):189–210
Budak H, Hussain B, Khan Z, Ozturk NZ, Ullah N (2015) From genetics to functional genomics: improvement in drought signaling and tolerance in wheat. Front Plant Sci 6:1012
Busch W, Wunderlich M, Schöffl F (2005) Identification of novel heat shock factor-dependent genes and biochemical pathways in Arabidopsis thaliana. Plant J 41(1):1–14
Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT (2007) A heat-inducible transcription factor HsfA2 is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiol 143(1):251–262
Chauhan H, Khurana N, Agarwal P, Khurana P (2011) Heat shock factors in rice (Oryza sativa L.): genome-wide expression analysis during reproductive development and abiotic stress. Mol Genet Genomics 286(2):171–187
Chauhan H, Khurana N, Agarwal P, Khurana JP, Khurana P (2013) A seed preferential heat shock transcription factor from wheat provides abiotic stress tolerance and yield enhancement in transgenic Arabidopsis under heat stress environment. PLoS ONE 8:11
Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17(1):268–281
de Pinto MC, Locato V, Paradiso A, De Gara L (2015) Role of redox homeostasis in thermo-tolerance under a climate change scenario. Ann Bot 116(4):487–496
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defence against lipid peroxidation. J Exp Bot 32(1):79–91
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ, Alharby H, Wu C, Wang D, Huang J (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Fortunati A, Barta C, Brilli F, Centritto M, Zimmer I, Schnitzler JP, Loreto F (2008) Isoprene emission is not temperature-dependent during and after severe drought-stress: a physiological and biochemical analysis. Plant J 55(4):687–697
Gai YP, Ji XL, Lu W, Han XJ, Yang GD, Zheng CC (2011) A novel late embryogenesis abundant like protein associated with chilling stress in Nicotiana tabacum cv bright yellow-2 cell suspension culture. Mol Cell Proteomics 10(11):M111.010363
Galvez S, Merida-Garcia R, Camino C, Borrill P, Abrouk M et al (2018) Hotspots in the genomic architecture of field drought responses in wheat as breeding targets. Funct Integr Genomics 19:295–309
Goyal K, Walton LJ, Tunnacliffe A (2005) LEA proteins prevent protein aggregation due to water stress. Biochem J 388(1):151–157
Guo M, Lu JP, Zhai YF, Chai WG, Gong ZH, Lu MH (2015) Genome-wide analysis expression profile of heat shock factor gene family (CaHsfs) and characterisation of CaHsfA2 in pepper (Capsicum annuum L.). BMC Plant Biol 15(1):151
Harwood WA, Smedley MA (2009) Barley transformation using biolistic techniques. In: Jones HD, Shewry PR (eds) Transgenic wheat, barley and oats. Humana Press, Totowa, pp 125–136
Heerklotz D, Doring P, Bonzelius F, Winkelhaus S, Nover L (2001) The balance of nuclear import and export determines the intracellular distribution and function of tomato heat stress transcription factor HsfA2. Mol Cell Biol 21(5):1759–1768
Hill JE, Hemmingsen SM (2001) Arabidopsis thaliana type I and II chaperonins. Cell Stress Chaperones 6(3):190–200
Hu W, Hu G, Han B (2009) Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci 176(4):583–590
Hu Y, Han Y, Wei W, Li Y, Zhang K, Gao Y, Zhao F, Feng J (2015) Identification isolation and expression analysis of heat shock transcription factors in the diploid woodland strawberry Fragaria vesca. Front Plant Sci 6:736
Huang DW, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37(1):1–13
Huang YC, Niu CY, Yang CR, Jinn TL (2016) The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol 172(2):1182–1199
Hussain B, Lucas SJ, Ozturk L, Budak H (2017) Mapping QTLs conferring salt tolerance and micronutrient concentrations at seedling stage in wheat. Sci Rep 7:15662
Hwang SM, Kim DW, Woo MS, Jeong HS, Son YS, Akhter S, Choi GJ, Bahk JD (2014) Functional characterization of Arabidopsis HsfA6a as a heat-shock transcription factor under high salinity and dehydration conditions. Plant Cell Environ 37(5):1202–1222
Ikeda M, Mitsuda N, Ohme-Takagi M (2011) Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible Hsfs but positively regulate the acquired thermotolerance. Plant Physiol 157(3):1243–1254
Ishikawa A, Tanaka H, Nakai M, Asahi T (2003) Deletion of a chaperonin 60β gene leads to cell death in the Arabidopsis lesion initiation 1 mutant. PlantCell Physiol 44:255–261
Jacob P, Hirt H, Bendahmane A (2017) The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol J 15(4):405–414
Janda T, Khalil R, Tajti J, Pál M, Darkó É (2019) Responses of young wheat plants to moderate heat stress. Acta Physiol Plant 41(8):137
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6(13):3901–3907
Jung KH, An G (2012) Application of MapMan and RiceNet drives systematic analyses of the early heat stress transcriptome in rice seedlings. J Plant Biol 55(6):436–449
Jung KH, Ko HJ, Nguyen MX, Kim SR, Ronald P, An G (2012) Genome-wide identification and analysis of early heat stress responsive genes in rice. J Plant Biol 55(6):458–468
Karimi M, Inzé D, Depicker A (2002) GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7(5):193–195
Kaushal N, Gupta K, Bhandhari K, Kumar S, Thakur P, Nayyar H (2011) Proline induces heat tolerance in chickpea (Cicer arietinum L.) plants by protecting vital enzymes of carbon and antioxidative metabolism. Physiol Mol Biol Plants 17(3):203
Kim SR, Yang JI, An G (2013) OsCpn60α1 encoding the plastid chaperonin 60α subunit is essential for folding of rbcL. Mol Cells 35(5):402–409
Kotak S, Larkindale J, Lee U, von Koskull-Döring P, Vierling E, Scharf KD (2007a) Complexity of the heat stress response in plants. Curr Opin Plant Biol 10(3):310–316
Kotak S, Vierling E, Baumlein H, von Koskull-Döring P (2007b) A novel transcriptional cascade regulating expression of heat stress proteins during seed development of Arabidopsis. Plant Cell 19(1):182–195
Kovacs D, Kalmar E, Torok Z, Tompa P (2008) Chaperone activity of ERD10 and ERD14 two disordered stress-related plant proteins. Plant Physiol 147(1):381–390
Kudla J, Batistic O, Hashimoto K (2010) Calcium signals: The lead currency of plant information processing. Plant Cell 22(3):541–563
Kumar M, Busch W, Birke H, Kemmerling B, Nurnberger T, Schoffl F (2009) Heat shock factors HsfB1 and HsfB2b are involved in the regulation of Pdf1.2 expression and pathogen resistance in Arabidopsis. Mol Plant 2(1):152–165
Kumar S, Kaur R, Kaur N, Bhandhari K, Kaushal N, Gupta K, Bains TS, Nayyar H (2011) Heat-stress induced inhibition in growth and chlorosis in mungbean (Phaseolus aureus Roxb.) is partly mitigated by ascorbic acid application and is related to reduction in oxidative stress. Acta Physiol Plant 33(6):2091
Lamaoui M, Jemo M, Datla R, Bekkaoui F (2018) Heat and drought stresses in crops and approaches for their mitigation. Front Chem 6:26
Leprince O, Buitink J (2010) Desiccation tolerance: from genomics to the field. Plant Sci 179(6):554–564
Li X (2011) Infiltration of Nicotiana benthamiana protocol for transient expression via Agrobacterium. Bio Protocol 1(14):e95
Li C, Chen Q, Gao X, Qi B, Chen N, Xu S, Chen J, Wang X (2005) AtHsfA2 modulates expression of stress responsive genes and enhances tolerance to heat and oxidative stress in Arabidopsis. Sci China Ser C Life Sci 48(6):540–550
Liu HC, Liao HT, Charng YY (2011) The role of class A1 heat shock factors (HSFA1s) in response to heat and other stresses in Arabidopsis. Plant Cell Environ 34(5):738–751
Mann DGJ, Lafayette PR, Abercrombie LL, Parrott WA, Stewart CN (2011) pANIC: A versatile set of Gateway-compatible vectors for gene overexpression and RNAi-mediated down-regulation in monocots. In: Stewart Jr CN, Touraev A, Citovsky V, Tzfira T (eds) Plant transformation technologies. Blackwell Publ., Hoboken, pp 161–168
Mano J, Kanameda S, Kuramitsu R, Matsuura N, Yamauchi Y (2019) Detoxification of reactive carbonyl species by glutathione transferase tau isozymes. Front Plant Sci 10:487
Mansoor S, Naqvi FN (2013) Effect of heat stress on lipid peroxidation and antioxidant enzymes in mung bean (Vigna radiata L.) seedlings. Afr J Biotechnol 12(21):3196–3203
Mishra SK, Tripp J, Winkelhaus S, Tschiersch B, Theres K, Nover L, Scharf KD (2002) In the complex family of heat stress transcription factors HsfA1 has a unique role as master regulator of thermotolerance in tomato. Genes Dev 16(2):1555–1567
Mittal D, Chakrabarti S, Sarkar A, Singh A, Grover A (2009) Heat shock factor gene family in rice: genomic organization and transcript expression profiling in response to high temperature low temperature and oxidative stresses. Plant Physiol Biochem 47(9):785–795
Morales D, Rodríguez P, Dell'Amico J, Nicolas E, Torrecillas A, Sánchez-Blanco MJ (2003) High-temperature preconditioning and thermal shock imposition affects water relations gas exchange and root hydraulic conductivity in tomato. Biol Plant 47(2):203
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880
Nover L, Bharti K, Döring P, Mishra SK, Ganguli A, Scharf KD (2001) Arabidopsis and the heat stress transcription factor world: how many heat stress transcription factors do we need? Cell Stress Chaperones 6(3):177
Patel RK, Jain M (2012) NGS QC toolkit: a toolkit for quality control of next generation sequencing data. PLoS ONE 7(2):e30619
Pulido P, Llamas E, Rodriguez-Concepcion M (2017) Both Hsp70 chaperone and Clp protease plastidial systems are required for protection against oxidative stress. Plant Signal Behav 12(3):e1290039
Qu AL, Ding YF, Jiang Q, Zhu C (2013) Molecular mechanisms of the plant heat stress response. Biochem Biophys Res Commun 432(2):203–207
Reddy ASN, Ali GS, Celesnik H, Day IS (2011) Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 23(6):2010–2032
Reddy PS, Kishor PBK, Seiler C, Kuhlmann M, Eschen-Lippold L, Lee J, Reddy MK, Sreenivasulu N (2014) Unraveling regulation of the small heat shock proteins by the heat shock factor HvHsfB2c in barley: its implications in drought stress response and seed development. PLoS ONE 9(3):1–16
Risk JM, Selter LL, Chauhan H, Krattinger SG, Kumlehn J, Hensel G, Viccars LA, Richardson TM, Buesing G, Troller A, Lagudah ES, Keller B (2013) The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnol J 11(7):847–854
Saade S, Negrão S, Plett D, Garnett T, Tester M (2018) Genomic and genetic studies of abiotic stress tolerance in barley. In: Stein N, Muehlbauer GJ (eds) The barley genome. Springer, Cham, pp 259–286
Scharf KD, Berberich T, Ebersberger I, Nover L (2012) The plant heat stress transcription factor (Hsf) family: structure function and evolution. Biochim Biophys Acta Gene Regul Mech 1819(2):104–119
Schutzendubel A, Nikolova P, Rudolf C, Polle A (2002) Cadmium and H2O2-induced oxidative stress in Populus × canescens roots. Plant Physiol Biochem 40(6–8):577–584
Sivamani E, Bahieldin A, Wraith JM, Al-Niemi T, Dyer WE, Ho THD, Qu R (2000) Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci 155(1):1–9
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126(1):45–51
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25(9):1105–1111
Tricase C, Amicarelli V, Lamonaca E, Rana RL (2018) Economic analysis of the barley market and related uses. In: Tadele Z (ed) Grasses as food and feed. IntechOpen, London. https://doi.org/10.5772/intechopen.78967
Vandenabeele S, Vanderauwera S, Vuylsteke M, Rombauts S, Langebartels C, Seidlitz HK, Zabeau M, Van Montagu M, Inzé D, Van Breusegem F (2004) Catalase deficiency drastically affects gene expression induced by high light in Arabidopsis thaliana. Plant J 39(1):45–58
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61(3):199–223
Wang G, Cai G, Xu N, Zhang L, Sun X, Guan J, Meng Q (2019) Novel Dnaj protein facilitates thermotolerance of transgenic tomatoes. Int J Mol Sci 20(2):1–19
Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Sandermann H, Langebartels C (2002) Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ 25(6):717–726
Wu Z, Liang J, Wang C, Zhao X, Zhong X, Cao X, Li G, He J, Yi M (2018) Overexpression of lily HsfA3s in Arabidopsis confers increased thermotolerance and salt sensitivity via alterations in proline catabolism. J Exp Bot 69(8):2005–2021
Xu D, Duan X, Wang B, Hong B, Ho THD, Wu R (1996) Expression of a late embryogenesis abundant protein gene HVA1 from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110(1):249–257
Xue GP, Sadat S, Drenth J, McIntyre CL (2014) The heat shock factor family from Triticum aestivum in response to heat and other major abiotic stresses and their role in regulation of heat shock protein genes. J Exp Bot 65(2):539–557
Xue GP, Drenth J, McIntyre CL (2015) TaHsfA6f is a transcriptional activator that regulates a suite of heat stress protection genes in wheat (Triticum aestivum L) including previously unknown Hsf targets. J Exp Bot 66(3):1025–1039
Yoshida T, Ohama N, Nakajima J, Kidokoro S, Mizoi J, Nakashima K, Maruyama K, Kim J-M, Seki M, Todaka D, Osakabe Y, Sakuma Y, Schöffl F, Shinozaki K, Yamaguchi-Shinozaki K (2011) Arabidopsis HsfA1 transcription factors function as the main positive regulators in heat shock-responsive gene expression. Mol Genet Genomics 286(5–6):321–332
Zhu Y, Wang Z, Jing Y, Wang L, Liu X, Liu Y, Deng X (2009) Ectopic over-expression of BhHsf1 a heat shock factor from the resurrection plant Boea hygrometrica leads to increased thermotolerance and retarded growth in transgenic Arabidopsis and tobacco. Plant Mol Biol 71(4–5):451
Acknowledgements
This work was supported by an early career research award (ECR/2016/000084) and core research Grant (CRG/2019/002579) by SERB-DST, Government of India, to HC. AKP, SKM, and PS thank MHRD and RC thanks UGC for providing the research fellowships. PS also thanks the FRQNT, University of Quebec, Trois-Rivières Quebec, Canada for providing the research internship. We also thank Melodie B Plourde and Claire Letanneur for help in Nicotiana infiltration assays.
Funding
This work is supported by research Grants ECR/2016/000084 and CRG/2019/002579 by SERB-DST, Government of India to HC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Communicated by Dorothea Bartels.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Poonia, A.K., Mishra, S.K., Sirohi, P. et al. Overexpression of wheat transcription factor (TaHsfA6b) provides thermotolerance in barley. Planta 252, 53 (2020). https://doi.org/10.1007/s00425-020-03457-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-020-03457-4